PUBLIC DOMAIN, HTTPS://COMMONS.WIKIMEDIA.ORG/W/INDEX.PHP?CURID=2580464
PUBLIC DOMAIN, HTTPS://COMMONS.WIKIMEDIA.ORG/W/INDEX.PHP?CURID=2580464
Monoclonal Antibodies and Their Impact on Oral Healthcare
As more patients begin taking biologics and their generic counterparts, oral health professionals need to be knowledgeable about their indications, adverse reactions, and risk of interactions with other medications in order to provide safe and effective care.
This course was published in the January 2021 issue and expires January 2024. The author has no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Define monoclonal antibodies (mAbs) and how they work.
- Identify the therapeutic indications of mAbs.
- List the adverse effects and reactions caused by the use of mAbs
Comprising a large and fast-growing group of medications with diverse therapeutic targets, monoclonal antibodies (mAbs) have a wide-ranging variety of current and potential therapeutic applications. The first mAb was generated in 1975, and the first mAb muromonab-CD3, a mouse antibody used to prevent kidney transplant rejection, was approved by the United States Food and Drug Administration (FDA) in 1986.1 Progress in biotechnology allowed for development of partially and then fully human mAbs, which reduce the risk of immune response and permit much wider use.1,2 The field of mAbs has progressively grown since its introduction: 2018 had the greatest number of FDA approvals—12—and, by August 2020, five new mAbs were approved, with 17 more under review.3,4
Along with vaccines, blood/blood components, tissues, cells, and other substances of biological origin, mAbs are “biologics” produced by complex biotechnological methods overseen by the FDA. Like typical chemotherapeutic agents, biological products undergo the rigorous process of approval, monitoring, and post-market surveillance, ensuring their safety and effectiveness and helping to detect adverse reactions not identified during clinical trials. And, despite their specificity to the intended cells and tissues defined as “targeted therapies” and generally good tolerability, mAbs are not without adverse effects, including oral and peri-oral—some severe enough to warrant therapy discontinuation.5,6 As the variety, number, and therapeutic indications for mAbs continue to increase,3,7,8 the chance of encountering patients using these biologics (or the growing number of their generic versions, known as biosimilar products) in the dental setting will grow. Understanding the conditions being treated and their manifestations, therapeutic and adverse reactions of the medication regimens, and interactions with other drugs and supplements is vital to providing safe dental and periodontal treatment. Awareness of oral and peri-oral adverse reactions to mAbs will help in differential diagnosis, while detailed communication with the prescribing providers will aid in determining and providing appropriate care.
DEFINITION OF MONOCLONAL ANTIBODIES
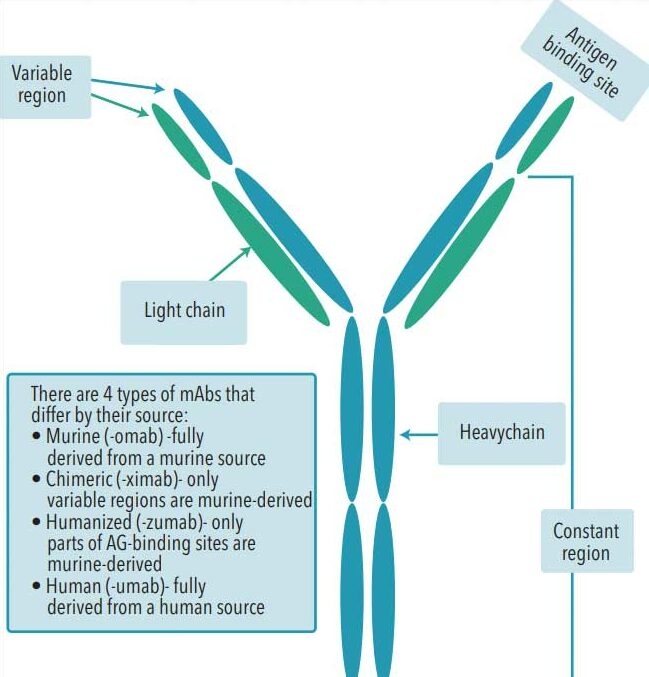
Antibodies are immunoglobulin (Ig) molecules, which are proteins of five different classes—IgA, IgD, IgE, IgG, and IgM—produced by the B-lymphocytes as a defense mechanism against foreign substances (eg, microorganisms, allergens) and those produced by the host (such as cancer cells or normal healthy cells in case of autoimmune reactions). In a typical immune reaction, the immunoglobulins are produced by many different B-cells with only slight differences in their binding site or affinity to the antigen; these immunoglibulins are called polyclonal. In contrast, monoclonal immunoglobulins are all produced by a single B-lymphocyte clone with identical structures and antigen-binding characteristics. Sftructurally, mAbs are IgG proteins, the most abundant and the smallest of the Igs with a molecular weight of ~150 kDa. There are four IgG subclasses in humans.7 Consisting of two pairs of the heavy and light chains, mAbs have constant and variable regions. The unique hypervariable region at the end of variable region determines mAb specificity and interaction with the target site of the antigen. Immonogenecity of the mAbs decreases depending on the origin of the constant and variable/hypervariable regions from fully murine to chimeric (~33% mouse), humanized (~90% human), and fully human.2,6,9
The large size and complex molecular structure of mAbs may inhibit their distribution to the sites of action and, thus, their efficacy. To overcome this, smaller fragments (Fabs) consisting of the mAbs variable site and portions of light and heavy chains have been developed. These fragment antibodies and single-chain antibodies improve pharmacokinetic properties and efficiency of dissemination of the drugs to their targets.1,10 In addition, due to their “lock and key” specificity, mAbs can be used for targeted delivery of other drug molecules to the intended cells by creating conjugates of the drug/toxin and the appropriate mAb.1,9,10 The conjugates of mAbs with cytotoxic drugs or radionuclides (Table 1), enzymes, and plant/bacterial toxins ensure their direct delivery to the intended targets and minimize potential damage to healthy cells.9
quantities.
mAbs exert several mechanisms of action and some drugs work by more than one mode, triggering multiple receptor and cellular interactions.6,9 Neutralization of the target can be accomplished by mAbs blocking action against the receptor or its binding molecule, referred to as receptor- or ligand-antagonism. Examples of mAbs that work by blocking include adalimumab, which targets tissue necrosis factor (TNF) involved in inflammation (used in treatment of rheumatoid arthritis [RA], Crohn disease, and psoriasis), and omalizumab, which targets IgG produced in allergic reactions and asthma (used in long-term treatment of severe asthma attacks).9
This mechanism of action is also used in reversing drug actions. For instance, Fab idarucizumab binds to the oral anticoagulant dabigatran, neutralizing it in case of bleeding. It is the first reversal agent for the novel oral anticoagulants, whose availability allows for a wider use of anticoagulants, such as dabigatran as a safer alternative to warfarin.11 Finally, neutralization is the mechanism of action for the treatment and prophylaxis of viral and bacterial infections, targeting agents such as respiratory syncitial virus, human immunodeficiency virus, rabies virus, Clostridium difficile, and Bacillus anthrasis (Table 1).3,4,12 The COVID-19 pandemic also prompted robust investigation into the potential use of mAbs against SARS-CoV-2 by blocking the interaction of the virus’ spike proteins with key cellular receptors in the human tissues.13
Blocking the target signaling pathway is the mechanism of action for many mAbs used in cancer treatment. Upon binding to the target tumor cell receptor, mAb will affect the downstream signaling and intracellular messaging, resulting in inhibition of cell growth or proliferation, or induction of the programmed cell death (apoptosis).9 However, this action is not limited to cancer treatment. Three mAbs have been approved for migraine prevention (erenumab, galcanezumab, eptinezumab) since 2018, and all target calcitonine gene-related peptide receptor located on the neuron cell surface and whose activation induces intracellular cascade, leading to propagation of the pain impulse.4,14
Following their binding to the target cell, mAbs can recruit immune cells to generate the antibody-dependent cell-mediated cytotoxicity (ADCC). This mechanism, as well as similar immune-mediated complement-dependent cytotoxicity (CDC) reaction, leads to the death of the target cell via lysis.9 These interactions involving the host immune system usually work together with neutralizing function of the mAbs and are used in cancer immunotherapies. Cetuximab is such a drug, targeting epithelial growth factor receptor (EGFR) expressed on the surface of cancer cells. It uses all three modes: blocking, ADCC, and CDC in treatment of colorectal and head and neck cancers.9
mAb drugs are administered by subcutaneous, intramuscular, or intravenous injection because their oral bioavailability is very low.7,15 Subcutaneous administration is used most frequently due to its convenience and possibility of self-adminstration.7 As mAbs are large proteins with heavy molecular sizes, they have a slower distribution with peak plasma concentrations usually 3 days to 7 days post-administration. Unlike most typical small-molecule drugs, mAbs are not eliminated by the kidneys, instead they are degraded to peptides/amino acids by intracellular lysosomal metabolism and then either recycled for protein synthesis or eliminated though the kidneys as smaller molecules.3 This explains their longer half-lives and provides an advantage of administration on a weekly/biweekly or monthly schedule.7,15
Produced by several complex biotechnological processes, mAbs require extensive purification to ensure their biological activity, therapeutic quality, and safety.1–3 Unfortunately, this process is very costly, which may render them out of the financial reach of many patients, despite their therapeutic potential.1,2
THERAPEUTIC INDICATIONS
The number and variety of therapeutic applications of mAbs continue to grow with the identification of cellular and receptor targets that have been considered “undruggable” by traditional chemotherapeutics.10 For many diseases, such as RA, mAbs are the standard of care, while in cancer therapy, they are often used in conjunction with traditional treatment methods such as radiation and chemotherapy.10,12,16 For conditions marked by intense pain, such as migraine, or painful unsightly lesions as in psoriasis, mAb immunotherapies can significantly improve patients’ quality of life by reducing the number and intensity of episodes or improving appearance.14,17 Table 1 lists some of mAbs’ many therapeutic indications. While some conditions can be treated with multiple mAbs, others can be used in management of more than one condition.
ADVERSE EFFECTS AND REACTIONS
Generally, due to their high specificity to intended targets, mAbs are well tolerated, and adverse effects are typically mild—ranging from skin reactions at the site of injection to flu-like symptoms.3,6,9 However, some patients experience adverse reactions severe enough to necessitate drug discontinuation, and some are life threatening.6
Most severe acute reactions following mAbs administration include immediate or delayed-onset anaphylaxis (type I hypersensitivity); serum sickness (type III hypersensitivity reaction); tumor lysis syndrome (TLS); and cytokine release syndrome (CRS), also known as cytokine storm. In fact, mAbs can provoke all four types of hypersensitivity reactions.18 Immune-mediated reactions to mAbs are not entirely preventable with reduction of animal-derived antibody sequence. An immune response against fully-human mAbs can also be initiated due to differences in protein sequence.6,18 This risk is higher in chimeric and humanized mAbs; for example, omalizumab—used to treat acute asthma attack—can trigger anaphylactic reaction in 0.1% to 0.2% of patients.6 TLS is a life-threatening reaction to mAbs used in anti-neoplastic therapy and it is directly related to destroying cancer cells whose lysis generates a systemic inflammatory response. It can lead to overwhelming ionic imbalance and progress to acute renal failure, cardiac arrhythmias, and death.18 Finally, CRS is an acute complication resulting from the release of various pro-inflammatory cytokines. In one report, systemic inflammatory reaction to the massive cytokine release progressed from headache, malaise, nausea, and hypotension to lung injury, renal failure, and disseminated intravascular coagulation, followed by cardiovascular shock and acute respiratory distress syndrome. CRS highlighted the potential danger associated with mAbs and led to changes in determining the initial doses of mAbs in human clinical studies.6 Still, CRS is not entirely preventable, and presents a serious potential complication.
Infectious diseases due to immunosuppression associated with mAb therapies can be prevented but not eliminated by screening prior to mAb therapy. A reactivation of latent human polyomavirus-2 in the central nervous system can lead to progressive multifocal leukoencephalopathy—a rapidly progressing demyelinating disease that resembles multiple sclerosis and is usually fatal.18
Thrombocytopenia is another drug-induced acute immune complication that can be caused by mAbs. Its prevalence increases with repeat administrations, and it can be fatal.6,18 As immunomodulating agents, mAbs can also cause autoimmune conditions such as lupus-like syndromes, thyroid disease, and autoimmune colitis, though these are rare.6,18 While used as therapeutic agents in oncology,9,12,18 increased cancer risk is associated with some mAbs.6 The role of the immune system in identifying and targeting malignant cells and the immunosuppressive mechanism of action of mAbs helps explain this association.3,6
Other adverse reactions can be directly related to the intended function of mAbs. For example, bevacizumab—which targets vascular endothelial growth factor (VEGF) controlling development of blood vessels—blocks VGRF function in malignant tumors and is used in the treatment of breast, lung, colorectal, and other susceptible cancers. However, its effect is beyond the tumor, restricting angiogenesis in healthy tissues as well.9 Similarly, cetuximab and panitumumab, used in the treatment of solid tumors, block EGFR located in the epithelial tissues. Since these agents block EGF pathways in healthy tissues as well as tumors, they commonly cause negative dermatological and mucosal—including oral/perioral—effects.6,19 When administered with radiation- or chemotherapy, these mAbs can exacerbate mucositis associated with those therapies.19
ORAL/PERIORAL ADVERSE EFFECTS AND COMPLICATIONS
Some mAbs cause less frequent but concerning complications, such as oral lichenoid drug-induced reactions or eruptions (OLDRs)20–24 and medication-related osteonecrosis of the jaw (MRONJ).25,26 OLDRs are indistinguishable from idiopathic lichen planus, and histological differences are not definitive.22,24 Painful OLDRs may be unilateral and found in less typical locations (labial, palatal mucosa) than lichen planus (bilateral buccal mucosa, tongue).22 They can appear from several days to weeks into treatment with mAbs.20–23 Differential diagnosis is based primarily on a thorough medical/drug history, and improvement or disappearance of lesions following drug discontinuation confirms diagnosis.22,24 However, this must be done in consultation with prescribing physician and only if an acceptable substitute drug is available. Topical or systemic corticosteroids may be prescribed to facilitate healing and the complete disappearance of the lesions may take weeks or months after drug discontinuation.20,21,23
The clinical presentation of MRONJ related to mAbs is no different from that caused by bisphosphonate use. Necrotic lesions most frequently appear on the mandible following surgery, tooth extractions, implant placements, or spontaneously, from a few weeks to more than a year after beginning mAb therapy.26 Several mAbs with different mechanisms of action have shown association with MRONJ, including denosumab used in osteoporosis prevention; bevacizumab used in cancer treatment; and adalimubab and rituximab used in the management of RA, psoriasis, ulcerative colitis, and other immune-mediated conditions (Table 1).25,26 Pathogenesis of MRONJ in the case of denosumab and immunomodulatory mAbs is not well understood, while anti-VEGF agents affect the bone due to their inhibition of angiogenesis and reduction of vascularization of bone.26 As there is no effective treatment for MRONJ, its prevention is key, and patients may be advised to complete dental/periodontal treatments prior to initiation of immunotherapies, or, if possible, to postpone them.26 As always, thorough evaluation of the patients’ medication use and, if necessary, consultation with their medical providers will help in diagnosis and management of their oral/perioral complications.
CONCLUSION
Targeted immunotherapies with mAbs represent one of the fastest growing areas of drug development today. Oral health professionals’ understanding of their therapeutic indications and effects, as well as general and oral adverse effects of various mAbs, is key to providing comprehensive, collaborative patient care.
REFERENCES
- Liu JKH. The history of monoclonal antibody development—progress, remaining challenges and future innovations. Ann Med Surg. 2014;3:113–116.
- Van Hoecke L, Roose K. How mRNA therapeutics are entering the monoclonal antibody field. J Transl Med. 2019;17:54.
- Castelli MS, McGonigle P, Hornby PJ. The pharmacology and therapeutic applications of monoclonal antibodies. Pharmacol Res Perspect. 2019;7:6.
- The Antibody Society. Antibody Therapeutics Approved or in Regulatory Review in the EU or US. Available at: antibodysociety.org/resources/approved-antibodies. Accessed December 21, 2020.
- United States Food and Drug Administration. What Are “Biologics?” Questions and Answers. FDA. Available at: fda.gov/about-fda/center-biologics-evaluation-and-research-cber/what-are-biologics-questions-and-answers. Accessed December 21, 2020.
- Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJT. The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov. 2010;9:325–338.
- Ovacik M, Lin K. Tutorial on monoclonal antibody pharmacokinetics and its considerations in early development. Clin Transl Sci. 2018;11:540–552.
- Kaplon H, Muralidharan M, Schneider Z, Reichert JM. Antibodies to watch in 2020. mAbs. 2020;12:1703531.
- Suzuki M, Kato C, Kato A. Therapeutic antibodies: their mechanisms of action and the pathological findings they induce in toxicity studies. J Toxicol Pathol. 2015;28:133–139.
- Slastnikova TA, Ulasov AV, Rosenkranz AA, Sobolev AS. Targeted intracellular delivery of antibodies: the state of the art. Front Pharmacol. 2018;9:1208.
- Jarrett JB, Gimbar RP. Idarucizumab (Praxbind) for Dabigatran (Pradaxa) anticoagulant reversal. Am Fam Physician. 2017;95:798–800.
- Drewe E, Powell RJ. Clinically useful monoclonal antibodies in treatment. J Clin Pathol. 2002;55:81–85.
- Marovich M, Mascola JR, Cohen MS. Monoclonal antibodies for prevention and treatment of COVID-19. JAMA. 2020;324:131–132.
- American Migraine Foundation. CGRP Antibodies and Receptor Blockers for Migraine. Available at: americanmigrainefoundation.org/resource-library/cgrp-antibodies-and-receptor-blockers/ Accessed December 21, 2020.
- Keizer RJ, Huitema ADR, Schellens JHM, Beijnen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49:493–507.
- Nelson AL, Dhimolea E, Reichert JM. Development trends for human monoclonal antibody therapeutics. Nat Rev Drug Discov. 2010;9:767–774.
- National Psoriasis Foundation. What Is Psoriasis, What Causes It and What Are Your Treatment Options? Available at: psoriasis.org/about-psoriasis Accessed December 21, 2020.
- Baldo BA. Adverse events to monoclonal antibodies used for cancer therapy. Oncoimmunology. 2013;2:10.
- Chmieliauskaite M, Stojanov I, Saraghi M, Pinto A. Oral adverse events associated with targeted cancer therapies. Gen Dent. 2018;66:26–31.
- Kuten-Shorrer M, Hochberg EP, Woo S-B. Lichenoid mucosal reaction to rituximab. Oncologist. 2014;19:e12–13.
- Lee M, Seetharamu N. An atypical presentation of lichen planus-like reaction from pembrolizumab. Case Rep Dermatol Med. 2019;2019:4065437.
- Rice PJ, Hamburger J. Oral lichenoid drug eruptions: their recognition and management. Dent Update. 2002;29:442–447.
- Thompson JM, Cohen LM, Yang CS, Kroumpouzos G. Severe, ulcerative, lichenoid mucositis associated with secukinumab. JAAD Case Rep. 2016;2:384–386.
- Bariş E, Sengüven B, Tüzüner T, Gültekin SE. Oral lichenoid lesions related to drugs: review of clinicopathological features and differential diagnosis. Eur J Inflamm. 2014;12:217–225.
- Lombard T, Neirinckx V, Rogister B, Gilon Y, Wislet S. Medication-related osteonecrosis of the jaw: new insights into molecular mechanisms and cellular therapeutic approaches. Stem Cells Int. 2016;2016:8768162.
- Eguia A, Bagan L, Cardona F. Review and update on drugs related to the development of osteonecrosis of the jaw. Med Oral Patol Oral Cir Bucal. 2020;25:e71–e83.
From Dimensions of Dental Hygiene. January 2021;19(1):36-39.