
Instrument Sterilization
A systematic approach to dental instrument processing.
This course was published in the January 2010 issue and expires January 2013. The author has no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Set-up an instrument processing area.
- Understand how to transport, clean, package, and sterilize dental instruments.
- Describe the different methods of dental instrument sterilization.
- Effectively and safely store dental instruments.
INSTRUMENT PROCESSING AREA
Instrument processing should be done in an area separate from patient treatment. The equipment, work, and storage areas should be designed to promote a one-directional flow of instruments from dirty to clean to sterile. The Centers for Disease Control and Prevention (CDC) recommends that a central processing area be designated with four defined sections for receiving, cleaning, and decontamination; preparation and packaging; sterilization; and storage.1
Surfaces have the potential to transmit infectious agents, such as influenza viruses and Staphylococcus aureus, therefore the disinfection of surfaces in the instrument processing area is just as important as in patient treatment areas. The surface should be sprayed or wiped once to clean, then again to disinfect to make sure that disinfection is successful. Spray disinfectants or disinfectant wipes can be used to keep surfaces clean.
TRANSPORTING USED INSTRUMENTS
The container should be shallow enough that it will not require someone to reach in to remove the instruments since this could present a puncture injury hazard. If a deeper container is necessary, OSHA recommends the use of strainer type baskets to hold the instruments and the use of forceps to remove them.3
INSTRUMENT CLEANING
Cleaning is a critical step in instrument processing. According to the CDC Guideline for Disinfection and Sterilization in Healthcare Facilities 2008: “Items must be cleaned using water with detergents or enzymatic cleaners before processing. Cleaning reduces the bioburden and removes foreign material (ie, organic residue and inorganic salts) that interferes with the sterilization process by acting as a barrier to the sterilization agent.”4 The use of a presoak solution may increase the effectiveness of the mechanical cleaning device, especially if instruments cannot be cleaned before the debris dries.5
 The most effective methods for cleaning dental instruments are performed with the use of mechanical devices such as ultrasonic instrument washers or washer/disinfectors.6 Ultrasonic washers use sound waves to create cavitations in the solutions, which help loosen debris. A cleaning solution should be used that is intended for ultrasonic cleaning. Household detergents, disinfectants, or other materials should not be used. When using an ultrasonic washer, follow the manufacturer’s instructions for cleaning time. Although little contamination occurs during the ultrasonic cleaning process, the risk of contamination can be diminished further by putting a lid on the ultrasonic washer when it is activated.7 Protective eyewear/ goggles and a face mask or a face shield should be worn in addition to protective clothing to protect against spray and splash. Heavy duty gloves should be donned throughout the cleaning, drying, and packaging procedures. Upon removal from the ultrasonic washer, instruments need to be thoroughly rinsed and carefully dried, taking care to avoid unnecessary handling of sharp instruments.
The most effective methods for cleaning dental instruments are performed with the use of mechanical devices such as ultrasonic instrument washers or washer/disinfectors.6 Ultrasonic washers use sound waves to create cavitations in the solutions, which help loosen debris. A cleaning solution should be used that is intended for ultrasonic cleaning. Household detergents, disinfectants, or other materials should not be used. When using an ultrasonic washer, follow the manufacturer’s instructions for cleaning time. Although little contamination occurs during the ultrasonic cleaning process, the risk of contamination can be diminished further by putting a lid on the ultrasonic washer when it is activated.7 Protective eyewear/ goggles and a face mask or a face shield should be worn in addition to protective clothing to protect against spray and splash. Heavy duty gloves should be donned throughout the cleaning, drying, and packaging procedures. Upon removal from the ultrasonic washer, instruments need to be thoroughly rinsed and carefully dried, taking care to avoid unnecessary handling of sharp instruments.
Instrument washers use a combination of cleaning solutions, hot water, and pressure to remove debris from instruments. In addition to instrument washers, some manufacturers make devices that are designated as washer/disinfectors (Figure 1). These thermal disinfectors are calibrated to ensure a water temperature of a duration adequate to highlevel disinfect the instruments. Packaging and heat sterilizing all heat stable critical and semicritical instruments are still necessary.1
Hand scrubbing—although not prohibited by OSHA—is discouraged because mechanical devices are more effective for cleaning instruments than hand scrubbing. Hand scrubbing increases the amount of handling of contaminated dental instruments by personnel and thus increases the opportunities for accidental exposure to blood or other potentially infectious materials.6
INSTRUMENT PACKAGING
 The selection of instrument packaging is a decision that should involve consideration of the type of sterilization process, the nature and type of patient care items being sterilized, the methods of storage, and any special needs of the dental practice. Wraps, pouches, and tubing come in a variety of materials including paper, nylon, and plastic. Paper and plastic are usually safe for use in steam and unsaturated chemical sterilizers. Nylon pouches or tubing are often indicated with dry heat since paper may scorch and plastic can melt in the higher temperatures of a dry heat sterilizer.6
The selection of instrument packaging is a decision that should involve consideration of the type of sterilization process, the nature and type of patient care items being sterilized, the methods of storage, and any special needs of the dental practice. Wraps, pouches, and tubing come in a variety of materials including paper, nylon, and plastic. Paper and plastic are usually safe for use in steam and unsaturated chemical sterilizers. Nylon pouches or tubing are often indicated with dry heat since paper may scorch and plastic can melt in the higher temperatures of a dry heat sterilizer.6
INSTRUMENT STERILIZATION
The CDC provides specific recommendations for selecting which items must be sterilized and which items can be subjected to high-level disinfection only (Table 1). This recommendation asks the dental professional to identify instruments as semi-critical, critical, or noncritical based on the risk the use of each type presents to patient safety.8 Reusable critical and semi-critical patient care items should be heat sterilized between uses.1 Very few, if any, reusable intraoral items will fail to withstand the heat sterilization process. For items that will not withstand heat sterilization, evaluate first whether a heat stable or disposable alternative is available. If an alternative is not available, determine the appropriate method of processing, keeping in mind the recommendations for the three categories of patient care equipment. Some state dental boards have adopted the CDC Guidelines for Infection Control in Dental Health Care Settings into their state regulations—making the recommendations mandatory by state law.9
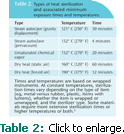 The three most common methods of sterilization used in dental offices are steam heat under pressure (autoclave), dry heat, and unsaturated chemical vapor.8 Each type of sterilization process has defined parameters for achieving sterilization (Table 2). With all methods of sterilization, personnel should review the manufacturer’s instructions for use, including type of packaging, loading instructions, placement of packs, and operation of the sterilizer.
The three most common methods of sterilization used in dental offices are steam heat under pressure (autoclave), dry heat, and unsaturated chemical vapor.8 Each type of sterilization process has defined parameters for achieving sterilization (Table 2). With all methods of sterilization, personnel should review the manufacturer’s instructions for use, including type of packaging, loading instructions, placement of packs, and operation of the sterilizer.
STEAM AUTOCLAVE
Steam autoclaves use a combination of saturated steam under pressure for a specific time period to effectively achieve sterilization. There are two methods of air evacuation used in steam sterilization: gravity displacement and prevacuum. In gravity displacement steam enters the sterilization chamber, displacing the air through a vent until the chamber is full of pressurized steam. A prevacuum sterilizer uses a vacuum pump to remove air from the chamber before injecting steam. This provides a more rapid introduction of steam and also allows the autoclave to pump the steam out of the chamber for the drying cycle. Overall, prevacuum autoclaves (with post-vacuum cycles) will process and dry instrument packs faster than most gravity autoclaves.4 To ensure proper functioning of prevacuum sterilizers, an air removal test should be performed periodically. If air is not completely removed before initiation of the sterilization cycle, it may result in failure of the autoclave to completely sterilize the contents.4 With both types of autoclaves the packs must be allowed to dry thoroughly before removing them from the chamber after sterilization. Wet packs are easier to tear—potentially compromising the sterility of the contents.6
Unwrapped, flash sterilization cycles are only indicated for special circumstances when items cannot be packaged and stored before use. According to the CDC Guideline for Disinfection and Sterilization in Healthcare Facilities 2008: “Flash sterilization should not be used for reasons of convenience, as an alternative to purchasing additional instrument sets, or to save time.”4
DRY HEAT
Dry heat sterilizers are usually popular in practices that use hinged instruments because of concern regarding the damage moisture may cause to the hinges. Dry heat sterilizers may be either static-air or forced air. Static-air relies on natural convection of the heated air to circulate through the chamber. In a forced-air system, air is circulated throughout the chamber, which allows a more rapid transfer (sometimes called rapid heat-transfer sterilization) of heat through the chamber, resulting in a shorter sterilization cycle.8
UNSATURATED CHEMICAL VAPOR
The unsaturated chemical vapor sterilizer functions in a manner similar to steam autoclaves with the primary difference being that a chemical agent is used to generate steam rather than water. This reduces the water moisture, providing a sterilization process that is less likely to result in corrosion of sensitive materials such as carbon steel. The chemical product is a formula that contains alcohol and formaldehyde as primary active ingredients. Proper ventilation is necessary to purge the vapors before opening the sterilizer at the end of the cycle. Purge cycles and filters are often built in to reduce residual vapors.10
STERILITY ASSURANCE
 The CDC Guideline for Disinfection and Sterilization in Healthcare Facilities 2008 states: “The delivery of sterile products for use in patient care depends not only on the effectiveness of the sterilization process but also on the unit design; decontamination, disassembling, and packaging of the device; loading the sterilizer; monitoring; sterilant quality and quantity; the appropriateness of the cycle for the load contents; and other aspects of device reprocessing.”4 Sterilization monitoring, an important element of sterility assurance, is a process by which the dental personnel ensure that both the mechanical and operator functions are successful in achieving sterilization. There are three main methods of sterilization monitoring: mechanical indicators, chemical indicators, and biological indicators.10
The CDC Guideline for Disinfection and Sterilization in Healthcare Facilities 2008 states: “The delivery of sterile products for use in patient care depends not only on the effectiveness of the sterilization process but also on the unit design; decontamination, disassembling, and packaging of the device; loading the sterilizer; monitoring; sterilant quality and quantity; the appropriateness of the cycle for the load contents; and other aspects of device reprocessing.”4 Sterilization monitoring, an important element of sterility assurance, is a process by which the dental personnel ensure that both the mechanical and operator functions are successful in achieving sterilization. There are three main methods of sterilization monitoring: mechanical indicators, chemical indicators, and biological indicators.10
Mechanical indicators are the gauges, tapes, or digital monitors that indicate time, temperature, and pressure. They may also indicate when a cycle is incomplete or there has been a mechanical failure of the sterilizer. These mechanical indicators help alert personnel to a problem with the sterilization cycle but are not a reliable indicator of instrument sterility.10
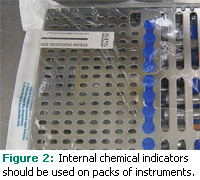
Chemical indicators are located on the outside or inside of sterilization pouches and may be embedded in packaging material or presented as paper. Some chemical indicators, such as autoclave tape, only measure one parameter of sterilization (eg, heat). Other chemical indicators, called multi-parameter indicators, are highly sensitive and measure all parameters (eg, heat, pressure, time). Multiparameter indicators are placed inside the pack of instruments along with the instruments and provide the highest level of assurity among the chemical indicators (Figure 2).
Biologic indicators (BI), also referred to as spore tests, provide the highest level of sterilization assurity if used properly. The CDC Guidelines for Disinfection and Sterilization in Healthcare Facilities 2008 advise: “Use BIs to monitor the effectiveness of sterilizers at least weekly with a Food and Drug Administration-cleared commercial preparation of spores…”4 More frequent use of a BI may be indicated, such as when processing implantable items.1,4,10 The implantable items should be isolated after sterilization until the BI results are obtained. Spores used in BI are specific to the different types of sterilization processes. It is important to select the appropriate BI for the type of sterilization process. Geobacillus stearothermophilus is the BI used to monitor steam autoclaves and unsaturated chemical vapor sterilizers. The spores of Bacillus atrophaeus are appropriate to monitor dry heat sterilizers.10 Inoculated with 106 spores either on a paper strip or in a vial of culture medium, BIs are placed in the sterilization cycle with a typical load and, when removed from the sterilizer, are either incubated on-site or sent to a professional laboratory for incubation. A control BI from the same lot as the test BI should be incubated with the test and should yield a positive result.10 The test BI should yield a negative result to indicate sterilization was achieved. A successful spore test is one in which all test spores are killed during the sterilization process.
STORAGE
Sterilized patient care items should remain in their sterile packaging until needed for patient care. Maintain a clean and dry storage location. Moisture can compromise the integrity of the pouches and wraps used for sterilization by causing them to tear.6 If instrument packs become wet, torn, or are otherwise compromised, the items need to be repackaged and sterilized again. As long as the items have been kept clean, dry, and the packaging is intact, the instruments are safe for use. Dating packages for routine resterilization is not necessary, although it may be useful for identifying suspect packs of instruments in the event of a recall due to sterilization failure as indicated by a positive BI. With steam sterilization, objects other than implantable objects probably do not need to be recalled because of a single positive spore test unless the sterilizer or the sterilization procedure is defective as determined by maintenance personnel or inappropriate cycle settings. If additional spore tests remain positive, consider the items nonsterile and recall and reprocess the items for the implicated load or loads.1,4
CONCLUSIONS
The processing of patient care items requires a systematic approach. Following well-defined steps in a logical order helps reinforce good practices by all personnel involved in the process. Evaluating current practices, identifying areas that need adjustment or improvement, and then implementing improved procedures are the keys to developing an appropriate process. Continuous reinforcement through training and quality assurance/ monitoring practices helps promote a safe environment for patient care.
REFERENCES
- Kohn WG, Collins AS, Cleveland JL, et al. Guidelines for infection control in dental health-care settings—2003. MMWR Recomm Rep. 2003;52(RR-17):1-61.
- Occupational exposure to bloodborne pathogens; needlestick and other sharps injuries; final rule. Occupational Safety and Health Administration (OSHA), Department of Labor. Final rule; request for comment on the Information Collection (Paperwork) Requirements. Fed Regist. 2001;66:5318-5325.
- CPL 02-02-069 – CPL 2-2.69 – Enforcement Procedures for the Occupational Exposure to Bloodborne Pathogens. United States Department of Labor. Available at: www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=DIRECTIVES&p_id=2570. Accessed December 15, 2009.
- Rutala WA, Weber DJ, Healthcare Infection Control Practices Advisory Committee. Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008. Available at: www.cdc.gov/ncidod/dhqp/pdf/guidelines/Disinfection_Nov_2008.pdf. Accessed December 15, 2009.
- Walker N, Burke FJ, Palenik CJ. Comparison of ultrasonic cleaning schemes:a pilot study. Prim Dent Care. 2006;13:51-56.
- Harte JA, Molinari JA. Instrument processing and recirculation. Practical Infection Control in Dentistry. 3rd ed. Philadelphia: Lippincott, Williams & Wilkins; 2010:221-231.
- Bettner MD, Seiswanger MA, Miller CH, Palenik CJ. Effect of ultrasonic cleaning on microorganisms. Am J Dent. 1998;11:185-188.
- Miller CH, Palenik CJ. Sterilization, disinfection, and asepsis in dentistry. In: Block SS, ed. Disinfection, Sterilization and Preservation. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2001:1049-1068.
- Dofka C. Infection control and hazard management. In: Dofka SS, ed. Competency Skills for the Dental Assistant. Albany, NY: Delmar Publishing; 1996:44
- Harte JA, Molinari JA. Sterilization procedures and monitoring. Practical Infection Control in Dentistry. 3rd ed. Philadelphia: Lippincott, Williams & Wilkins; 2010:148-163.
From Dimensions of Dental Hygiene. January 2010; 8(1): 48-51.



