
Improve Oral Health with Mouthrinses
With mouthrinse use an already accepted practice by many patients, recommending a therapeutic product can help them also fight plaque bacteria and gingivitis.
This course was published in the May 2013 issue and expires May 2016. The author has no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Identify the difference between cosmetic and therapeutic mouthrinses.
- List the benefits of therapeutic mouthrinses.
- Discuss the mouthrinse ingredients that have proven efficacy and safety.
Achieving optimal health often involves changing longstanding habits, which is a challenging process. While dental professionals work to encourage healthier habits, patients may struggle with making changes. When planned habit changes can dovetail with current behaviors, implementation is more likely. If using a product or device is already an established behavior, it may be easier for patients to switch to a different type within the category than to adopt an entirely new behavior.
Many Americans use mouthrinses, although the exact reasons for purchase are unclear. Consumers may determine they need to do more than “just brush,” or they may use mouthrinse to appease the guilt of not flossing. Fresh breath is often a concern of patients, and advertising has established a link between mouthrinse use and the elimination of breath malodor. Cosmetic mouthrinses often promise benefits such as plaque removal, fresher breath, brighter teeth, and cleaner mouth. Although mouthrinses with these claims must demonstrate safety, their efficacy is not regulated by either the federal Food and Drug Administration (FDA) or the American Dental Association (ADA).
Therapeutic mouthrinses, on the other hand, address a disease process, such as gingivitis or caries. Currently, the use of mouthrinse to treat periodontitis is not supported by evidence.1 Mouthrinses with therapeutic claims must exhibit proof of their safety, and they need to obtain approval from the FDA. Consumer mouthrinses can also seek the ADA’s Seal of Acceptance as proof of the product’s safety and efficacy, but this is optional.
The sheer number of available mouthrinse products has created as much confusion for the average consumer as toothpaste selection. For both product categories, patients appreciate knowing which brand and type are optimal for their particular oral conditions, while also being cost-effective. Oral health care providers are viewed as product experts who can help patients sort through the options. For those who already have an established mouthrinse routine, recommending a more beneficial formulation won’t require adoption of a new behavior. Following a thorough oral health assessment, clinicians can offer an evidence-based and patient-centered mouthrinse regimen to address specific oral health needs.
BENEFITS OF MOUTHRINSES
Mouthrinsing is not a substitute for mechanical plaque biofilm control because it does not adequately penetrate plaque biofilm, supragingivally or subgingivally. However, the benefits of mechanical plaque control can be extended with the addition of a therapeutic mouthrinse.2 Mouthrinsing is a simple delivery system that allows a chemotherapeutic agent to reach either hard or soft tissues. Relatively inexpensive, mouthrinses are user-friendly because they require little time, effort, and skill, and most are over-the counter (OTC) purchases.
When the active agent must simply coat the intended surface, the mechanism and benefits are direct. However, impacting disease-associated bacteria is more challenging because microcolonies of bacteria within gingival areas are held together in the complex, sticky matrix of dental plaque biofilm (Figure 1). This biofilm is a highly structu
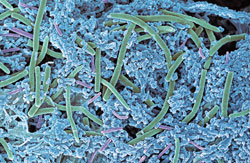
red ecosphere where a variety of interdependent bacterial populations can communicate and signal each other chemically about favorable conditions for continued attachment.
This biofilm matrix protects bacteria from antimicrobial agents. However, free-floating bacteria, not encased in biofilm matrix, are more susceptible to attack than those protected within biofilm. Consequently, mouthrinse antimicrobial agents can have the greatest impact on free-floating salivary bacteria in the early stages of attaching to teeth, gingiva, and other mucosal surfaces. When undisturbed, these free-floating supragingival bacteria, can “seed” gingival areas, where they may progress to complex periodontal disease-associated subgingival biofilm colonies that damage gingival and periodontal health. This explains why antibacterial mouthrinses may have their greatest impact against nonbiofilm bacteria that still cling to oral surfaces following mechanical plaque removal.
IMPACT ON GINGIVITIS AND PERIODONTITIS
Three factors often limit the efficacy of self-performed mechanical plaque biofilm removal: limited capacity of the available physical removal methods, less than optimal skill level, and inconsistent oral hygiene habits. The combination of these three factors, along with immune system considerations, hinder efforts to prevent and control gingivitis and periodontal diseases.
Mouthrinses are indicated for gingivitis, rather than periodontitis, because once an established biofilm overpowers the immune system causing periodontal pocketing/bone loss, the efficacy of an antibacterial mouthrinse depends on full access to the depth of periodontal pockets where the anaerobic pathogenic bacteria reside, as well as penetration of the complex biofilm. Because the fluid from rinsing penetrates no further than 1.5 mm subgingivally,3 it does not reach the intended bacterial populations. In addition, bacteria contained deep within biofilm are resistant to antimicrobial agents. The bacteria contributing to gingivitis, however, are located supragingivally and slightly subgingivally, so they are easily accessible to the mouthrinse solution.
Addressing gingivitis is critical because of its potential to progress to periodontitis. For products to claim therapeutic benefits in plaque and gingivitis reduction, the FDA requires positive evidence from randomized controlled trials (RCTs); similarly, the ADA Council on Dental Therapeutics stipulates that significant reductions in plaque and gingivitis should be demonstrated after 6 months of use in two studies when measured against a placebo control, rather than against baseline scores.4 The following mouthrinses all have this level of evidence to support their use.
DELMOPINOL
A mouthrinse containing 0.2% delmopinol hydrochloride was recently introduced in the United States as an OTC product. Delmopinol is not considered an antibacterial agent because rather than inactivating or killing bacteria, it interferes with formation of the gluelike polysaccharide that creates the plaque biofilm matrix, resulting in less adherence of bacteria to each other and to the teeth.5 Safety and efficacy have been validated. Studies have shown effective reductions in plaque (9.3% to 35% decrease), bleeding on probing (18% to 36%), and gingivitis (up to 18%).6–8 These RCTs demonstrated that delmopinol hydrochloride mouthrinse performed significantly better than a placebo, although not as effective as chlorhexidine. However, it was tested against a more concentrated chlorhexidine (0.2%), than what is used in the United States (0.12%).

Delmopinol mouthrinse has several advantages, including OTC availability, lower incidence of tooth staining than chlorhexidine, and low alcohol content (1.5%). It also creates a feeling of reduced plaque biofilm accumulation on the teeth that can be felt by the tongue. This can serve as a great teaching tool for patients to recognize the sensation of plaque-free teeth, which may not be as perceptible with other antibacterial mouthrinses that reduce bacterial counts, as opposed to impacting biofilm accumulation. This mouthrinse may be an excellent choice for patients concerned about plaque biofilm accumulation, but especially for heavy biofilm formers, those in orthodontic treatment, and for periodontal maintenance patients. Instructions should include rinsing for the prescribed 30 seconds only in order to limit food taste alteration immediately after use.
ESSENTIAL OIL
Essential oil mouthrinse is an OTC product that has been in use for more than 100 years. Its beneficial action results from disruption of the cell wall and inhibition of bacterial enzymes. This is facilitated by two phenol-related essential oils, thymol and eucalyptol, which are combined with menthol and methylsalicaylate. A long history has established safety with no development of opportunistic bacteria. Seven RCTs conducted over 6 months with unsupervised oral hygiene compared to a placebo or negative control have shown plaque reductions from 14.9% to 36.1% and gingivitis reductions from 9.4% to 35.9%, with greatest reductions seen when compared to a negative control.9–15 When the accompanying oral hygiene is standardized, even greater reductions are demonstrated.16–17 Essential oil mouthrinse provides plaque and gingivitis benefits when used with unsupervised brushing, compared to control or placebo, although it is not as effective as chlorhexidine due to chlorhexidine’s high substantivity.
The alcohol content in the original formula of essential oil mouthrinse may be contraindicated for patients with alcoholism or those recovering from it. Patients taking disulfiram (Antabuse®), a drug indicated for the treatment of alcoholism, or metronidazole (Flagyl®), an antibiotic, should not use an essential oil rinse, and it is contraindicated for children younger than 12. Interestingly, the zero alcohol formulation does not carry the same antigingivitis designation as the original formula, which holds the ADA Seal of Acceptance.
CETYLPYRIDINIUM CHLORIDE
Within the past several years, an OTC mouthrinse containing cetylpyridinium chloride (CPC) was reformulated at a higher concentration and with greater bioavailability to combat plaque and gingivitis than the original, which was marketed as a cosmetic breath freshener. The antiplaque/antigingivitis formulation (0.07% CPC), which also contains stannous fluoride, penetrates the bacterial cell membrane, resulting in leakage of cell components and bacterial cell death.18 Clinical trials lasting 6 months have shown plaque reductions of 16% to 28%, bleeding reduced from 27% to 67%, and reductions of 15% to 24% for gingivitis.19–20 The 0.07% CPC OTC mouthrinse, which is alcohol free, has been favorably compared to the essential oil product for its plaque biofilm and gingival benefits.21
CHLORHEXIDINE
Prescription mouthrinses containing 0.12% chlorhexidine gluconate are available in both an alcohol free and an 11.6% alcohol formulation. Both formulations are equally effective and disrupt the bacterial cell membrane, causing precipitation of the cytoplasm and resulting in bacterial death.21 Chlorhexidine has greater substantivity, or staying power, than any of the other OTC mouthrinses, which extends its antibacterial effect, but also contributes to more extrinsic tooth staining.
Extensive research over many years has documented the antiplaque and antigingivitis efficacy of this mouthrinse. Plaque reductions of 35% to 61% and gingivitis reductions of 37% to 40% have been shown.22–24 Studies comparing chlorhexidine mouthrinse to essential oil and delmopinol rinses showed that rinsing with chlorhexidine achieved the greatest plaque and gingivitis reductions.25–28 Deemed safe and effective, chlorhexidine helps to resolve gingivitis until the patient can establish consistent oral self-care habits to physically remove plaque biofilm. Adolescents in orthodontic treatment, who often exhibit poor oral hygiene, maintain high carbohydrate diets, and experience hormonal influences that raise the risk of gingivitis, may particularly benefit from a chlorhexidine mouthrinse.
CONCLUSIONS
Because so many patients already use a mouthrinse, recommending a therapeutic product that best meets their needs capitalizes on an established behavior. This may encourage improvement without the challenge of changing self-care habits. Therapeutic mouthrinse is a user-friendly component of a self-care regimen that does not require complex dexterity skills.
REFERENCES
- Ciancio, SG. The making of a mouthrinse. Dimensions of Dental Hygiene. 2008;6(11):36-37.
- Hughes P. An adjunct to mechanical plaque removal. Dimensions of Dental Hygiene.2006;4(4):32–34.
- Braun RE, Ciancio, SG. Subgingival deliveryby an oral irrigation device. J Periodontol.1992;63:469–472.
- Council on Dental Therapeutics. Guidelines for acceptance of chemotherapeutic products for the control of supragingival dental plaque and gingivitis. J Am Dent Assoc. 1986; 112:529-532
- Addy M, Moran J, Newcombe RG. Meta analyses of studies of 0.2% delmopinol mouthrinse as an adjunct to gingival health and plaque control measures. J Clin Periodontol. 2007;34:58–65.
- Lang NP, Hase JC, Grassi M et al. Plaque formation and gingivitis after supervised mouthrinsing with 0.2% delmopinol hydrochloride, 0.2% chlorhexidine digluconate and placebo for 6 months. Oral Dis. 1998;4:105–113.
- Hase JC, Attstrom R, Edwardsson S, Kelty E,Kisch J. 6-month use of 0.2% delmopinol hydrochloride in comparison with 0.2% chlorhexidine digluconate and placebo. (I). Effect on plaque formation and gingivitis. J Clin Periodontol. 1998; 25:746–753.
- Claydon N, Hunter L, Moran J, et al. 6-month home usage of 0.1% and 0.2% delmopinol mouthwashes. (I) Effect on plaque, gingivitis, supragingival calculus and tooth staining. J ClinPeriodontol. 1996;23:220–228.
- Lamster IB, Alfano MC, Seiger MC, GordonJM. The effect of Listerine antiseptic on reduction of existing plaque and gingivitis. Clin Prev Dent. 1983;5:12–16.
- Gordon JM, Lamster IB, Seiger MC. Efficacyof Listerine antiseptic in inhibiting the development of plaque and gingivitis. J Clin Periodontol. 1985;12:697–704.
- DePaola LG, Overholser CD, Meiller TF,Minah GE, Niehaus C. Chemo therapeutic inhibition of supragingival dental plaque andgingivitis development. J Clin Periodontol. 1989;16:311–315
- Grossman E, Meckel AH, Isaacs RL, et al. A clinical comparison of antibacterial mouthrinses: effects of chlorhexidine, phenolics, and sanguinarine on dental plaque and gingivitis. J Periodontol. 1989;60:435–440.
- Overholser CD, Meiller TF, DePaola LG,Minah GE, Niehaus C. Comparative effects of 2chemotherapeutic mouthrinses on thedevelopment of supragingival dental plaque
and gingivitis. J Clin Periodontol. 1990;17:575–579. - Charles CH, Sharma NC, Galustians HJ,Qaqish J, McGuire JA, Vincent JW. Comparativeefficacy of an antiseptic mouthrinse and anantiplaque/antigingivitis dentifrice. A six-monthclinical trial. J Am Dent Assoc. 2001;132:670–675.
- Charles CH, Mostler KM, Bartels LL, MankodiSM. Comparative antiplaque and antigingivitiseffectiveness of a chlorhexidine and an essential oil mouthrinse: 6-month clinical trial. JClin Periodontol. 2004;31:878–884.
- Triratana T, Rustogi KN, Volpe AR, DeVizioW, Petrone M, Giniger M. Clinical effect of anew liquid dentifrice containing triclosan/copolymer on existing plaque and gingivitis. J Am Dent Assoc. 2002;133:219–225.
- Sharma N, Charles CH, Lynch MC, et al.Adjunctive benefit of an essential oil-containing mouthrinse in reducing plaque and gingivitis inpatients who brush and floss regularly: a six month study. J Am Dent Assoc.2004;135:496–504.
- Sheie AA. Models of action of currently known chemical antiplaque agents other than chlorhexidine. J Dent Res. 1989; 68:1609–1616.
- Stookey GK, Beiswanger B, Mau M, IsaacsRL, Witt JJ, Gibb R. A 6-month clinical study assessing the safety and efficacy of two cetylpyridinium chloride mouthrinses. Am JDent. 2005;18:24A–28A.
- Mankodi S, Bauroth K, Witt JJ, et al. A 6-month clinical trial to study the effects of acetylpyridinium chloride mouthrinse on gingivitis and plaque. Am J Dent.2005;18:9A–14A.
- Albert-Kiszely A, Pjetursson BE, Salvi GE, etal. Comparison of the effects of cetylpyridiniumchloride with an essential oil mouth rinse ondental plaque and gingivitis—a six-month-randomized controlled clinical trial. J Clin Periodontol. 2007;34:658–667.
- Eldridge KR, Finnie SF, Stephens JA, et al.Efficacy of an alcohol-free chlorhexidine mouthrinse as an antimicrobial agent. J Prosthet Dent. 1998;80:685–690.
- Grossman E, Rieter G, Sturgenberger OP, et al. Six-month study of the effects of a chlorhexidine mouthrinse on gingivitis in adults. J Periodont Res. 1986;16(Suppl):33–43.
- Banting B, Bosam M, Bollmer B. Clinical effectiveness of a 0.12% chlorhexidinemouthrinse over 2 years. J Dent Res.1989;68:1716–1718
- Grossman E, Meckel AH, Isaacs RL, et al. Aclinical comparison of antibacteria lmouthrinses: effects of chlorhexidine,phenolics, and sanguinarine on dental plaque and gingivitis. J Periodontol. 1989;60:435–440.
- Overholser CD, Meiller TF, DePaola LG,Minah GE, Niehaus C. Comparative effects of 2chemotherapeutic mouthrinses on the development of supragingival dental plaque and gingivitis. J Clin Periodontol. 1990;17:575–579.
- Lang NP, Hase JC, Grassi M, et al. Plaque formation and gingivitis after supervised mouthrinsing with 0.2% delmopinol hydrochloride, 0.2% chlorhexidine digluconate and placebo for 6 months. Oral Dis. 1998;4:105–113.
- Charles CH, Mostler KM, Bartels LL, Mankodi SM. Comparative antiplaque and antigingivitis effectiveness of a chlorhexidine and an essential oil mouthrinse: 6-month clinical trial. J Clin Periodontol. 2004;31:878–884.
From Dimensions of Dental Hygiene. May 2013; 11(5): 66–69.



