
Encouraging Success
The dental hygienist plays an important role in the prevention, detection, and clinical management of peri-implant diseases.
This course was published in the May 2013 issue and expires May 2016. The author has no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Define peri-implant diseases and peri-implantitis and discuss the differences between the conditions.
- Explain the etiology of peri-implant diseases.
- Identify the role of the dental hygienist in the detection and prevention of peri-implant diseases.
INTRODUCTION
The April 2013 edition of the Journal of Periodontology published a paper “Peri-Implant Mucositis and Peri-Implantitis: A Current Understanding of Their Diagnoses and Clinical Implications,” which was developed under the direction of the Task Force on Peri-Implantitis and approved by the American Academy of Periodontology’s (AAP) Board of Trustees. The paper states that “routine monitoring of dental implants as part of a comprehensive periodontal evaluation and maintenance is essential” and recommends the following:
- Identify risk factors associated with peri-implant diseases.
- Establish radiographic baseline at the time of implant placement.
- Establish clinical and radiographic baseline at final prosthesis insertion.
- Employ methods that monitor implant health and determine inflammatory complications as part of an ongoing periodontal maintenance program.
- Establish an early diagnosis and intervention that will contribute to more effective management of periodontal diseases.
In addition to a comprehensive review of the literature, the paper provides additional information and background to help dental hygienists incorporate these guidelines into everyday clinical practice. Dr. Avila-Ortiz’s piece also emphasizes the importance of monitoring the health status of dental implants. As a supporter of dental professionals and their dedication to improving oral health, the Colgate-Palmolive Company is delighted to have provided an unrestricted educational grant to support the second article of this educational series “Encouraging Success” in collaboration with the AAP.
—Barbara Shearer, BDS, MDS, PhD
Director of Scientific Affairs
Colgate Oral Pharmaceuticals
Since the discovery of osseointegration and its subsequent application in dentistry,1 implant-supported restorations have been increasingly indicated for the treatment of total or partial edentulism. High survival rates (more than 95% for single-tooth replacement after 5 years), wide therapeutic versatility, and improved quality of life are some of the reasons behind the popularity of dental implants among patients and clinicians.2,3 In spite of the benefits associated with implant therapy, it is not without complications. A variety of biological and/or mechanical factors may affect implants, restorative components, and/or peri-implant tissues in different degrees of severity during the course of treatment. Among the multiple biological and mechanical conditions that may compromise implant therapy, peri-implantitis is increasing in prevalence and poses a significant challenge to clinicians. Dental hygienists see implant patients on a regular basis and, therefore, share the responsibility of recognizing problems that may affect implant therapy outcomes with the dentist.
DEFINITION
The terms “peri-implant disease” and “peri-implantitis” are commonly used to define any inflammatory condition affecting peri-implant tissues. However, the terms are not interchangeable because peri-implantitis falls within the broader family of peri-implant diseases. Peri-implant soft and hard tissues have a different response when subjected to microbial and/or mechanical challenges. In the Sixth European Workshop on Periodontology in 20084 the following definition for peri-implant mucositis was proposed: “an inflammatory lesion that resides in the peri-implant mucosa,” while peri-implantitis was defined as “an inflammatory lesion associated with the loss of implant supporting bone.” The definition of peri-implantitis has been recently revised and expanded in the Seventh European Workshop on Periodontology held in 2010: “an inflammatory lesion characterized by changes at the level of the crestal bone in conjunction with bleeding on probing with or without concomitant deepening of peri-implant pockets.”5
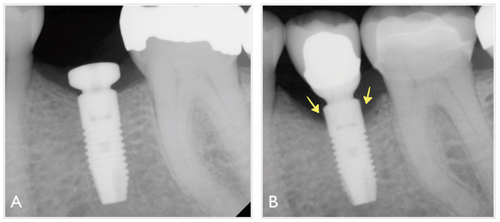
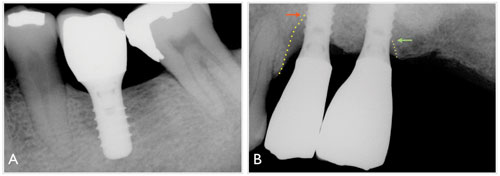
Similar to periodontitis, peri-implantitis is a destructive inflammatory process that usually presents increased pocket formation, swollen or red mucosal tissues, and loss of supporting bone (Figure 1). Both periodontitis and peri-implantitis are chronic conditions, and are typically asymptomatic in their courses, although acute episodes accompanied with pain may occur. However, other features establish clear differences between both diseases.
For example, while some degree of mobility (Miller’s grade 1 or 2) on a natural tooth is not necessarily an indicator of hopeless prognosis,6,7 an implant body exhibiting any degree of mobility is usually indicative of failure. Other distinctive characteristics of peri-implantitis are the pattern of the bony defects, which tend to present a circumferential, moat-like morphology (Figure 2), and the greater content of inflammatory infiltrate on peri-implantitis lesions as compared to peri-implant mucositis lesions.8 Also, observational studies suggest that subgingival calculus formation around im plants is less common than around teeth, which may be partially explained by the finding that, in general, peri-implantitis lesions progress at a faster rate than those affecting the periodontium.5
The epidemiology of peri-implant diseases varies greatly within the literature. In peri-implantitis, prevalence ranges from approximately 11% to 47% after 5 years to 15 years from implantation, according to recent reports.4,9 This may be because peri-implant diseases are a relatively new group of oral diseases and, as a result, a variety of definitions and clinical criteria have been proposed over the past two decades in the scientific literature. For this reason, Mombelli et al have suggested that current pooled estimations of the prevalence of peri-implantitis are likely inaccurate, given a potential overlapping between definitions from different studies. Long-term prospective studies collecting significant clinical and radiographic data at adequate intervals and with an appropriate sample size are required for a precise assessment of the prevalence of peri-implantitis. Unfortunately, these studies are not available.10
ETIOLOGY
The role of bacterial plaque in a susceptible host as the primary etiologic factor in peri-implant diseases is well documented in the literature.11–14 Similar to the pathogenesis of periodontal diseases, colonization by oral microbes of the mucosa surrounding dental implants triggers a cascade of biologic events that leads to the development of inflammatory processes modulated by the individual host’s immune response, which initially manifests as peri-implant mucositis.15 When left untreated, the inflammation may spread to the underlying osseous structures causing bone loss and, therefore, peri-implantitis.
Pontoriero et al conducted a study aimed at determining the clinical and microbiological features of experimental gingivitis as compared to experimental peri-implant mucositis. No significant differences were found in clinical and microbiological outcomes between experimentally induced peri-implant mucositis and gingivitis around natural teeth.16 This study suggested biofilm is the essential etiologic component leading to peri-implant mucositis, in a similar cause and effect relationship between the accumulation of bacterial plaque and the development of inflammation, as it was reported for gingivitis several decades ago.17 Recent investigations have added more pieces to the intriguing puzzle that is the microbiology of peri-implantitis, suggesting that, compared to periodontitis, the composition of the subgingival microbiota in peri-implant lesions is more complex, which may partially explain why the clinical management of these lesions is so challenging.18
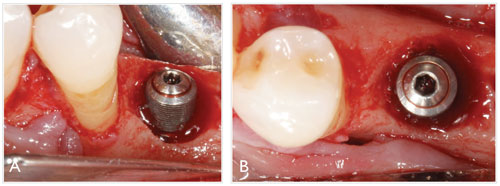
However, as stated by Mombelli and Decaillet, despite the fact that the primary etiology of peri-implantitis is bacterial plaque in a susceptible host, peri-implant infections may also arise opportunistically or progress faster due to nonmicrobial events that encourage the establishment of a submarginal pathogenic microbiota. 19 Some of these factors may include mechanical overload in the presence of existing inflammation, commonly due to excessive occlusal forces,20 and plaque-retentive elements. Within the latter category, poorly designed restorations and the presence of a residual luting agent around cement-retained restorations (Figure 3) are common iatrogenic factors that often lead to periimplant inflammation and eventually to bone loss, particularly in patients with a history of periodontitis.21,22
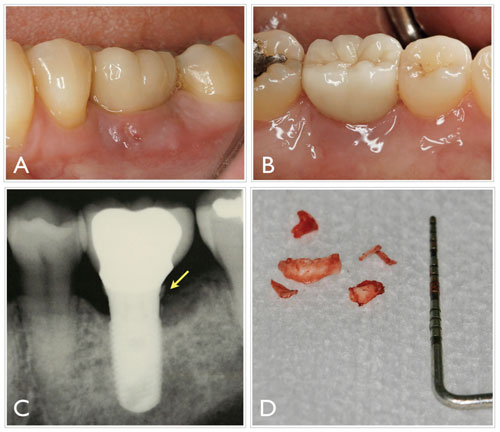
Peri-implant bone loss can occur at an early or late stage of treatment, before or after implant functional loading, and it may also progress continuously or remain stable after initial breakdown, where no further bone loss occurs.2 As such, not all cases of peri-implant crestal/marginal bone loss are periimplantitis. For example, bone loss is sometimes observed shortly after implant placement or functional loading around soft tissue level implants (those in which the restorative platform is intentionally placed coronally to the marginal bone level). In these instances, bone changes do not necessarily respond to an infectious or iatrogenic origin, but are the result of adaptive changes that take place in the marginal bone (Figure 4). The extent of these defects depends on the position of the implant platform (eg, how deep it is respective to the bone) and its macro-structural design (eg, these events are common around implants with a polished collar). On the other hand, bone loss around bone-level implants (those in which restorative platform is placed level with the marginal bone) is typically associated with a nonphysiologic and undesirable situation, as illustrated in Figure 1.
THE ROLE OF THE DENTAL HYGIENIST
Long-term survival of restored implants in the absence of symptoms (ie, no pain) and minimal progression of peri-implant bone loss was traditionally considered the definition of success.23 In contemporary oral implantology, some of these original paradigms have been substantially revised.2,24 Outcomes, such as patient quality of life, absence of mucosal inflammation, stable peri-implant bone levels, and optima esthetics, have been recognized as critical elements in the equation.25–27 Hence, an implant-supported restoration in a sustained and adequate state of peri-implant health, and esthetics has emerged as the gold standard for clinical success in oral implantology. The role of the dental hygienist in the early detection and prevention of peri-implantitis and other peri-implant diseases is crucial to minimize the initiation and progression of the disease.
Peri-implantitis is characterized by loss of supporting bone. However, marginal bone loss on standardized radiographs is not the only diagnostic criterion for peri-implantitis. The clinical detection of persistent signs of clinical inflammation (ie, mucosal erythema or redness, soft tissue swelling, bleeding on probing, and/or suppuration) and increasing pocket depth accompanying radiographic bone loss is necessary to establish a diagnosis of peri-implantitis. Pocket depths around implants tend to be deeper than around natural teeth. The absence of periodontal ligament, root cementum and, therefore, connective tissue attachment around dental implants is the most plausible reason behind this clinical characteristic of peri-implant tissues. However, this does not mean pocket depths greater than 5 mm should be accepted as normal. Factors such as the macrostructural design of the implant (eg, the shape, number of threads, platform position respective to the marginal bone, etc) and the prosthetic components, as well as the relative position of the implant platform, should be taken into account when evaluating the condition of the peri-implant tissues.
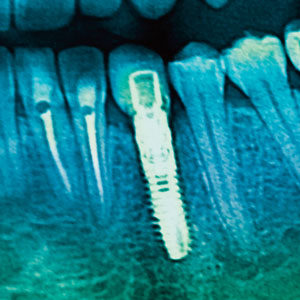
The use of a periodontal probe is fundamental to monitoring the status of the peri-implant tissues and to diagnose peri-implant diseases. In this regard, some concerns have been raised on the use of metal probes or scalers around implants and metal restorative components (eg, abutments and crowns) because the action of these instruments can produce surface alterations (eg, scratching) or leave metal and porcelain residues in the submarginal space that may predispose the accumulation of subgingival plaque and create a selective environment for more pathogenic bacteria.28 On the basis of these notions, the use of “softer” materials such as plastic, resin, or even titanium instruments for dental implant maintenance has been widely advocated in order to minimize surface alterations.29 The use of plastic covers for ultrasonic device tips has also been investigated.30 However, the use of plastic instruments
and tips is generally not recommended for implant instrumentation because they may be ineffective in removing calculus and/or cement, and surgical implant exposure and in vitro research has revealed plastic particles may adhere to implants when these instruments are employed.30,31 While the use of metal instruments may increase the roughness of abutment surfaces, no direct association between increased roughness and worsening of the peri-implant status has been established in controlled observational studies. Therefore, the effect of abutment surface alterations on the maintenance of peri-implant health has yet to be elucidated.32,33
A TEAM EFFORT
Peri-implant mucositis may be a reversible condition.34 For this reason, treatment of peri-implant mucositis is a generally straightforward process in the majority of cases, since it can be predictably controlled with prophylaxis, the modification of plaque retentive elements, and the implementation of adequate oral hygiene measures, which may include the use of specific, antimicrobial dentifrices.35,36 On the contrary, the clinical management of peri-implantitis represents a serious challenge for patients and clinicians alike.
Current peri-implantitis therapy protocols are markedly diverse, including nonsurgical therapy with or without anti-infective agents, occlusal adjustment, prosthesis modification, and surgical approaches for access and implant surface detoxification, which may also involve resective and/or regenerative therapy.37 Although some promising results have been reported,38 clinical protocols to deal with peri-implantitis are generally limited in their efficacy and predictability likely due to the complexity of this disease and the influence of multiple factors that are not fully understood yet.39,40 Additionally, the presence of a concomitant mucosal defect is an additional factor to consider for comprehensive treatment planning. Mucosal defects are not a recognized primary etiologic factor per se for peri-implant mucositis and periimplantitis, but rather a contributing factor, which often is a
direct consequence of progressing peri-implantitis. Furthermore, although commonly associated, not all the sites presenting periimplant mucosal defects also have concomitant peri-implant mucositis or peri-implantitis. Whether a minimum band of keratinized peri-implant mucosa is required for the maintenance of peri-implant health is a controversial topic.41,42 However, in particular cases with esthetic implications or if the persistence of the mucosal defect is detrimental for appropriate maintenance, surgical therapy to augment the keratinized mucosa may be indicated.43
For these reasons, although multiple treatment guidelines have been proposed, the indication of a particular therapy depends on the clinical presentation of the disease (eg, defect size) and the philosophy of the dentist. In extreme situations, the affected implants must be explanted and the missing bone has to be regenerated, which presents a very challenging clinical scenario. Additionally, there is a significant financial cost and morbidity (bone loss) usually associated with the treatment of peri-implantitis that can significantly affect the overall expense and the confidence of the patient in the originally proposed treatment.
The participation of the dental hygienist in identifying any significant changes in the status of the peri-implant tissues is essential in the prevention of peri-implantitis. Nonetheless, prevention and management of peri-implantitis requires a collective effort, that does not only involve the members of the dental team, but also the active participation of the patient, who must be appropriately instructed to perform adequate oral hygiene and understand the implications of treatment if peri-implantitis is diagnosed.
REFERENCES
- Branemark PI. Osseointegration and its experimental background. J Prosthet Dent. 1983;50:399–410.
- Albrektsson T, Buser D, Sennerby L. On crestal/marginal bone loss around dental implants. Int J OralMaxillofac Implants. 2012;27:736–738.
- Jung RE, Zembic A, Pjetursson BE, Zwahlen M, Thoma DS. Systematic review of the survival rate andthe incidence of biological, technical, and aesthetic complications of single crowns on implants reportedin longitudinal studies with a mean follow-up of 5 years. Clin Oral Implants Res. 2012;23(Suppl):2–21.
- Lindhe J, Meyle J. Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology.J Clin Periodontol. 2008;35:282–285.
- Lang NP, Berglundh T. Periimplant diseases: where are we now? Consensus of the Seventh EuropeanWorkshop on Periodontology. J Clin Periodontol. 2011;38(Suppl):178–181.
- Kwok V, Caton JG. Commentary: prognosis revisited: a system for assigning periodontal prognosis. J Periodontol. 2007;78:2063–2071.
- McGuire MK. Prognosis versus actual outcome: a long-term survey of 100 treated periodontal patient under maintenance care. J Periodontol. 1991;62:51–58.
- Gualini F, Berglundh T. Immunohistochemical characteristics of inflammatory lesions at implants. J Clin Periodontol. 2003;30:14–18.
- Koldsland OC, Scheie AA, Aass AM. Prevalence of peri-implantitis related to severity of the disease with different degrees of bone loss. J Periodontol. 2010;81:231–238.
- Mombelli A, Muller N, Cionca N. The epidemiology of peri-implantitis. Clin Oral Implants Res.2012;23(Suppl):67–76.
- Augthun M, Conrads G. Microbial findings of deep peri-implant bone defects. Int J Oral Maxillofac Implants. 1997;12:106–112.
- Leonhardt A, Renvert S, Dahlen G. Microbial findings at failing implants. Clin Oral Implants Res.1999;10:339–345.
- Quirynen M, Vogels R, Peeters W, van Steenberghe D, Naert I, Haffajee A. Dynamics of initial subgingivalcolonization of ‘pristine’ peri-implant pockets. Clin Oral Implants Res. 2006;17:25–37.
- Salcetti JM, Moriarty JD, Cooper LF, et al. The clinical, microbial, and host response characteristics ofthe failing implant. Int J Oral Maxillofac Implants. 1997;12:32–42.
- Berglundh T, Zitzmann NU, Donati M. Are peri-implantitis lesions different from periodontitis lesions?J Clin Periodontol. 2011;38(Suppl):188–202.
- Pontoriero R, Tonelli MP, Carnevale G, Mombelli A, Nyman SR, Lang NP. Experimentally induced periimplantmucositis. A clinical study in humans. Clin Oral Implants Res. 1994;5:254–259.
- Loe H, Theilade E, Jensen SB. Experimental Gingivitis in Man. J Periodontol. 1965;36:177–187.
- Koyanagi T, Sakamoto M, Takeuchi Y, Maruyama N, Ohkuma M, Izumi Y. Comprehensive microbiologicalfindings in peri-implantitis and periodontitis. J Clin Periodontol. 2013;40:218–226.
- Mombelli A, Decaillet F. The characteristics of biofilms in peri-implant disease. J Clin Periodontol.2011;38(Suppl):203–213.
- Naert I, Duyck J, Vandamme K. Occlusal overload and bone/implant loss. Clin Oral Implants Res.2012;23(Suppl):95–107.
- Linkevicius T, Puisys A, Vindasiute E, Linkeviciene L, Apse P. Does residual cement around implantsupportedrestorations cause peri-implant disease? A retrospective case analysis. Clin Oral Implants Res. 2012 Aug 8. [Epub ahead of print].
- Wilson TG Jr. The positive relationship between excess cement and peri-implant disease: a prospective clinical endoscopic study. J Periodontol. 2009;80:1388–1392.
- Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used denta limplants: a review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986;1:11–25.
- Misch CE, Perel ML, Wang HL, et al. Implant success, survival, and failure: the International Congressof Oral Implantologists (ICOI) Pisa Consensus Conference. Implant Dent. 2008;17:5–15.
- Annibali S, Bignozzi I, La Monaca G, Cristalli MP. Usefulness of the aesthetic result as a success criterion for implant therapy: a review. Clin Implant Dent Relat Res. 2012;14:3–40.
- Fürhauser R, Florescu D, Benesch T, Haas R, Mailath G, Watzek G. Evaluation of soft tissue around single-tooth implant crowns: the pink esthetic score. Clin Oral Implants Res. 2005;16:639–644.
- Papaspyridakos P, Chen CJ, Singh M, Weber HP, Gallucci GO. Success criteria in implant dentistry: a systematic review. J Dent Res. 2012;91:242–248.
- Quirynen M, Bollen CM, Papaioannou W, Van Eldere J, van Steenberghe D. The influence of titanium abutment surface roughness on plaque accumulation and gingivitis: short-term observations. Int J Oral Maxillofac Implants. 1996;11:169–178.
- Matarasso S, Quaremba G, Coraggio F, et al. Maintenance of implants: an in vitro study of titanium implant surface modifications subsequent to the application of different prophylaxis procedures. Clin Oral Implants Res. 1996;7:64–72.
- Mann M, Parmar D, Walmsley AD, Lea SC. Effect of plastic-covered ultrasonic scalers on titaniumimplant surfaces. Clin Oral Implants Res. 2012;23:76–82
- Ruhling A, Kocher T, Kreusch J, Plagmann HC. Treatment of subgingival implant surfaces with Tefloncoatedsonic and ultrasonic scaler tips and various implant curettes. Clin Oral Implants Res. 1994;5:19–29.
- Fakhravar B, Khocht A, Jefferies SR, Suzuki JB. Probing and scaling instrumentation on implant abutmentsurfaces: an in vitro study. Implant Dent. 2012;21:311–316.
- Louropoulou A, Slot DE, Van der Weijden FA. Titanium surface alterations following the use of differentmechanical instruments: a systematic review. Clin Oral Implants Res. 2012;23:643–658.
- Salvi GE, Aglietta M, Eick S, Sculean A, Lang NP, Ramseier CA. Reversibility of experimental periimplantmucositis compared with experimental gingivitis in humans. Clin Oral Implants Res. 2012;23:182–190.
- Ramberg P, Lindhe J, Botticelli D, Botticelli A. The effect of a triclosan dentifrice on mucositis in subjects with dental implants: a six-month clinical study. J Clin Dent. 2009;20:103–107.
- Sreenivasan PK, Vered Y, Zini A, et al. A 6-month study of the effects of 0.3% triclosan/copolymer dentifrice on dental implants. J Clin Periodontol. 2011;38:33–42.
- Aljateeli M, Fu JH, Wang HL. Managing peri-implant bone loss: current understanding. Clin Implant Dent Relat Res. 2012;14(Suppl): e109–e118.
- Froum SJ, Froum SH, Rosen PS. Successful management of peri-implantitis with a regenerative approach: a consecutive series of 51 treated implants with 3- to 7.5-year follow-up. Int J Periodontics Restorative Dent. 2012;32:11–20.
- Claffey N, Clarke E, Polyzois I, Renvert S. Surgical treatment of peri-implantitis. J Clin Periodontol. 2008;35:316–332.
- Renvert S, Roos-Jansåker AM, Claffey N. Non-surgical treatment of peri-implant mucositis and peri-implantitis: a literature review. J Clin Periodontol. 2008;35:305–315.
- Esposito M, Maghaireh H, Grusovin MG, Ziounas I, Worthington HV. Interventions for replacing missing teeth: management of soft tissues for dental implants. Cochrane Database Syst Rev. 2012;15;2.
- Lin GH, Chan HL, Wang HL. The Significance of Keratinized Mucosa on Implant Health: A Systematic Review. J Periodontol. 2013 Mar 1. [Epub ahead of print].
- Greenstein G, Cavallaro J. The clinical significance of keratinized gingiva around dental implants. Compend Contin Educ Dent. 2011;32:24–31.
From Dimensions of Dental Hygiene. May 2013; 11(5): 57–62.



