 SERGEYKLOPOTOV/ISTOCK/GETTY IMAGES PLUS
SERGEYKLOPOTOV/ISTOCK/GETTY IMAGES PLUS
Imaging Techniques for Patients with Cleft Lip and/or Palate
Various imaging modalities play an important role in the diagnosis and pre- and post-surgical phases of orofacial cleft therapy.
The most common congenital craniofacial anomalies are orofacial clefts, which include cleft lip and/or palate (CLP). These defects develop as a result of a complex interaction of genetic and environmental factors, resulting in a failure of the fusion of bony and soft tissue growth elements of the cranial and maxillofacial bones in utero. During this period, ultrasound and magnetic resonance imaging can assist in prenatal diagnosis, helping to prepare parents of children born with this condition.1–3 In addition, other forms of radiographic imaging, including cone-beam computed tomography (CBCT), are key tools for diagnosing and managing these defects. Clinicians also rely on the Tessier4 classification system, which is a comprehensive method of categorizing orofacial clefts based on craniofacial anatomy. The system describes 14 types of clefts according to their location in relation to eye and orbit. Facial clefts are numbered 0 through 7, and cranial clefts are numbered 8 through 14. Clefts of the lip, alveolus, and palate are numbered 1, 2, and 3 (Figure 1).
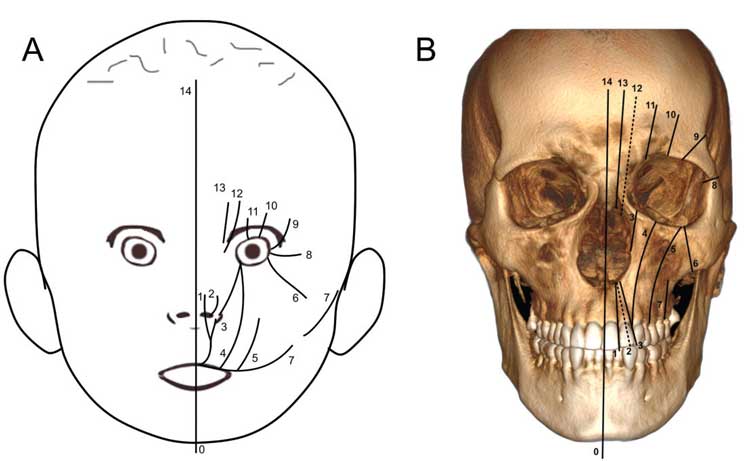
In this system, the most common orofacial clefts are those involving the upper lip, maxillary anterior alveolus (primary palate), and/or hard palate (secondary palate; groups 2 and 3). Other systems have been proposed to further describe CLP conditions, the most popular of which was suggested by Kernahan.5 From a treatment perspective, these conditions can be grouped into those that affect the lip with or without cleft palate, or cleft palate only. In this embryologically based system, the clefts are a result of specific anatomic derangements that fall along embryonic lines of fusion within the face. In the United States, there are an estimated 6.45 cases per 10,000 live births for cleft palate alone, and 10.89 for cleft lip with or without cleft palate.6
The comprehensive treatment of CLP is complex and involves addressing various surgical, dental, masticatory, esthetic, speech, and hearing problems from immediately after birth into early adulthood. Surgical intervention usually occurs in three or four stages: initial cleft lip repair at about 3 months of age; palatal cleft repair between 9 months and 12 months; pharyngeal flap procedure or pharyngoplasty (if required) between 3 years and 5 years of age; and primary palate repair (alveolar process) between ages 9 and 12.7 Patients are optimally managed through a multidisciplinary approach involving orthodontists, oral and maxillofacial surgeons, pediatric dentists, plastic surgeons, dermatologists, otolaryngologists, geneticists, and radiologists. In turn, these teams are supported by speech pathologists, social workers, and psychologists.8 Dental hygienists provide important educational, clinical, and advocacy support for patients with CLP and are also considered members of the support team.
Role of Imaging
As noted, radiographic imaging plays an important role in the diagnosis, monitoring, and pre- and post-surgical phases of treatment. Multiple radiographic procedures are performed on patients with CLP throughout childhood and adolescence. These may include panoramic, periapical, occlusal, and frontal or lateral cephalometric radiographs, as well as multidetector computed tomography and, more recently, cone-beam imaging. Compared to the general population, patients with CLP are exposed to a greater radiation detriment and therefore have greater radiation risk.9 Radiation exposure to patients with CLP, particularly children and adolescents, should therefore be as low as diagnostically acceptable (ALADA).10–12 This concept proposes that radiographic acquisition parameters should be selected to produce images of diagnostically acceptable quality suitable for task-specific interpretation, rather than just at the lowest settings.
More recently, Oenning et al13 provided guidelines on the appropriate use of cone-beam imaging in pediatric dentistry, with specific reference to patients with CLP. Because these patients are subject to serial imaging, Oenning and colleagues13 suggest imaging protocols include low-dose optimization techniques. For procedures involving CBCT, this would include the restriction of the field of view to the maxillary area, the use of half-scan modes (decreasing the number of basis projections), and reduction in exposure (such as milliampere-seconds). They propose a modification to ALADA that incorporates considerations of patient presentation and appropriate timing—namely, ALADAIP (as low as diagnostically acceptable being indication-oriented and patient-specific).
De Mulder et al14 recently proposed an optimized, comprehensive maxillofacial imaging protocol for patients with CLP (Table 1), with time points based on age: 6 years (start of treatment); 7 years to 9 years (or 10 years in conventional treatment planning); 15 years (presurgical records); and 20 years (end of the craniofacial growth period).8,14 Except for the initial radiographic examination, all imaging of patients with CLP is performed using CBCT at various fields of view and image quality exposure settings (eg, resolution and number of basis images).
Clinical Perspective
We have been involved in CBCT imaging of patients with CLP from referring clinicians since 2004. In the past 3 years, we have become an imaging referral center from an expanding group of external practitioners, including plastic surgeons and orthodontists. Most cases are referred to us for diagnostic assessment of patients with CLP at stages 2, 3, and 4. Because of the variability in patient timing, presentation, and potential complexity of the defect, we have adopted a comprehensive approach incorporating both image reformatting and interpretation to improve diagnosis and communication between referring practitioners.15,16
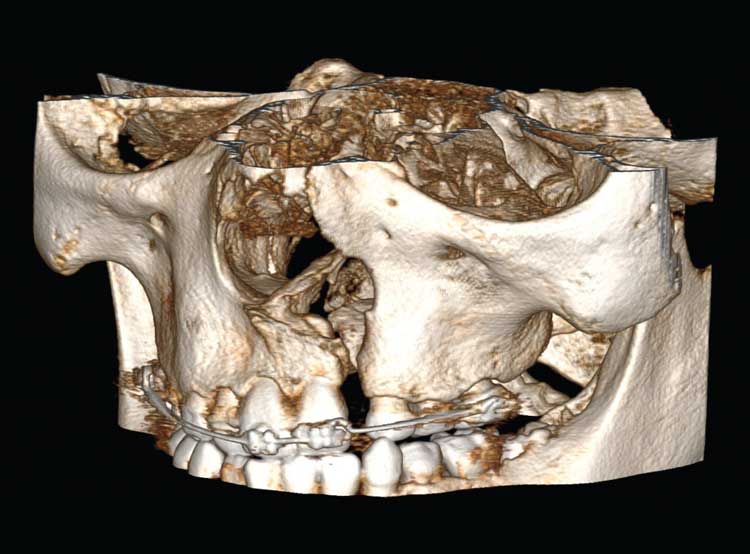
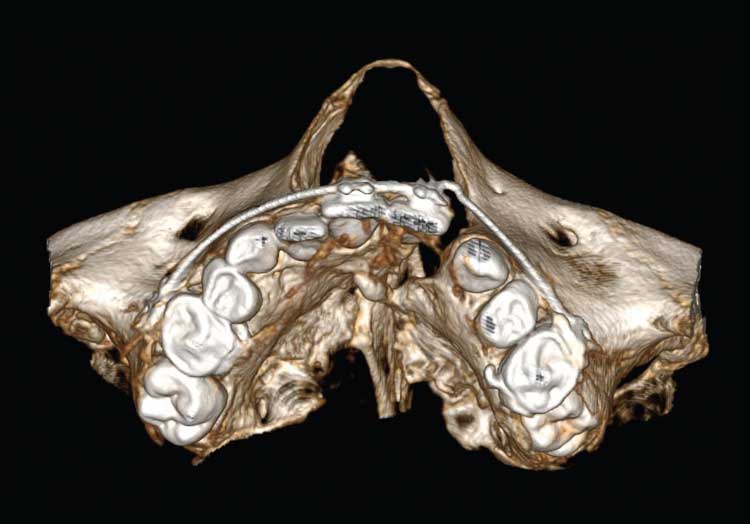
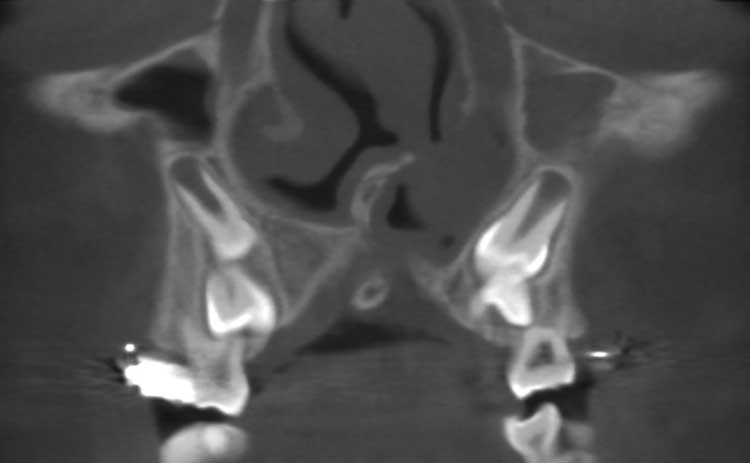
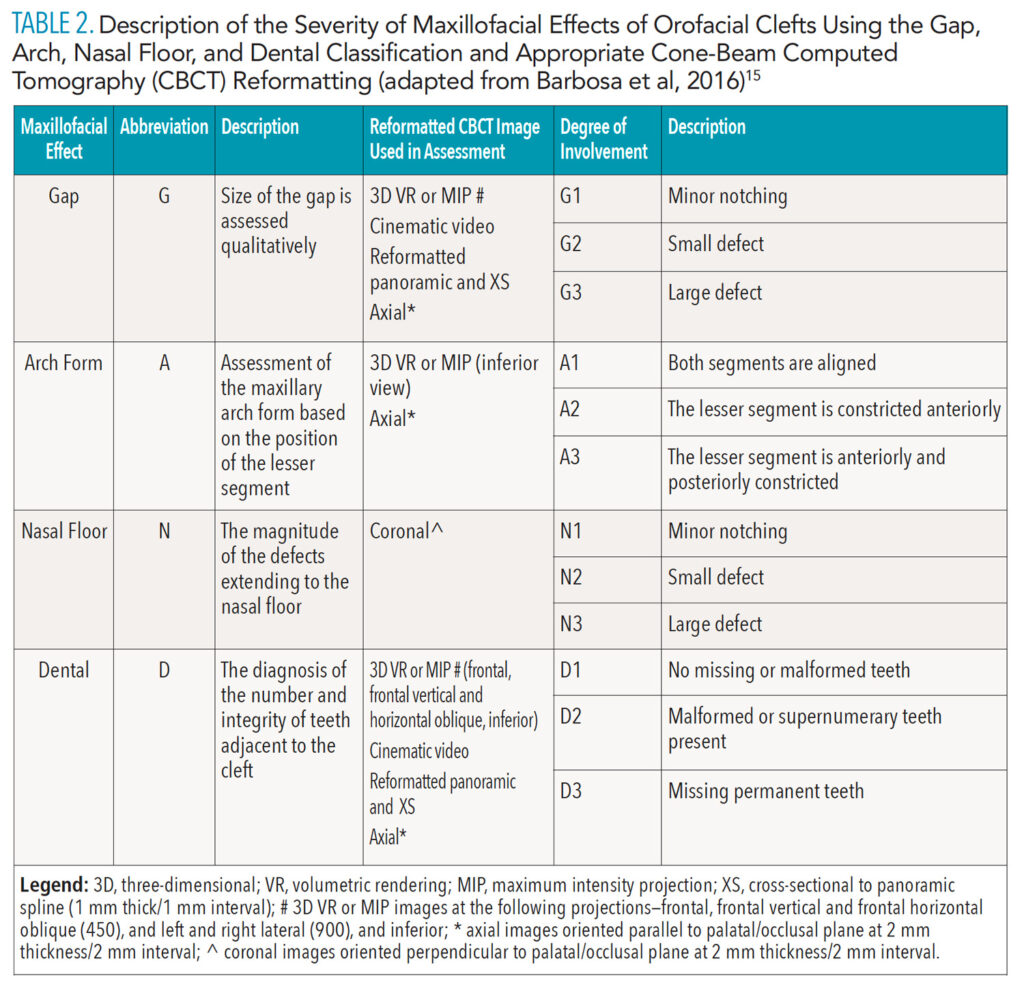
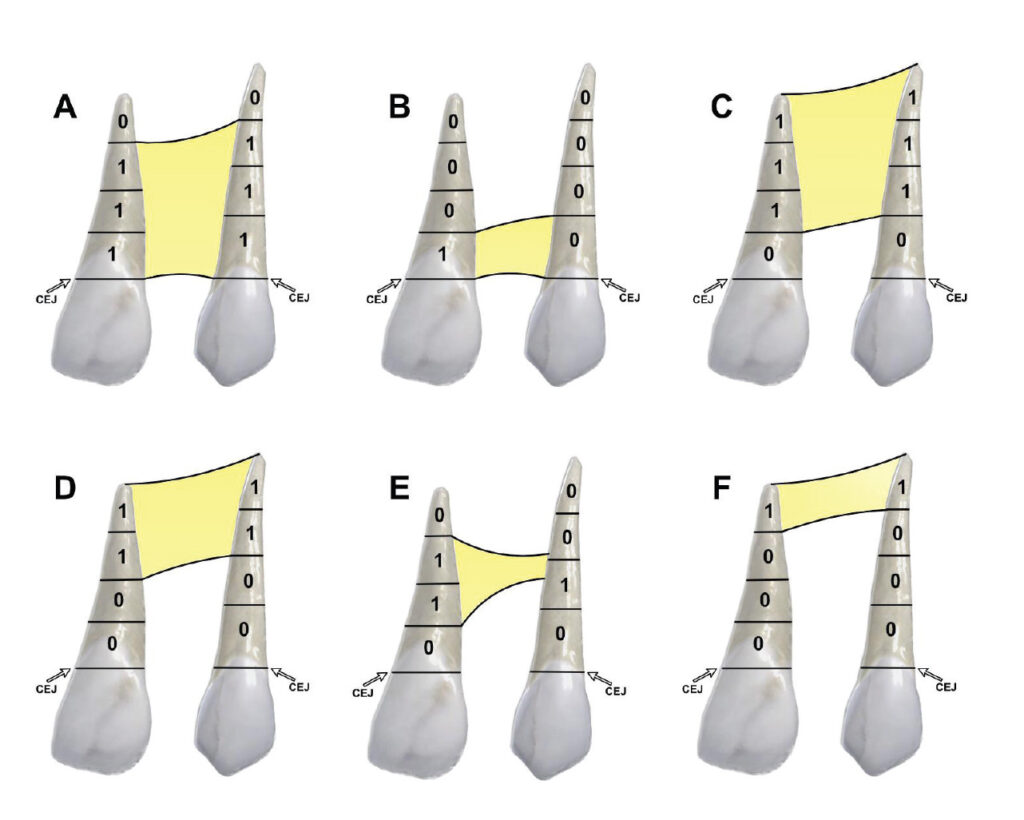
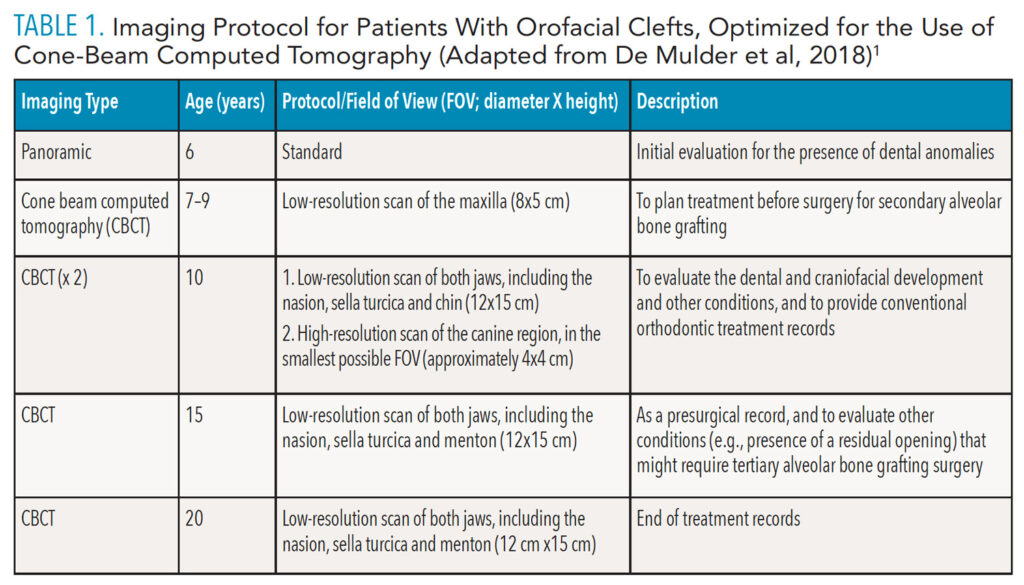 We describe the overall cleft defect and affected adjacent tissues on appropriately formatted CBCT images according to the gap, arch, nasal floor, and dental (GAND) classification15 (Table 2, and Figure 2 through Figure 4). This includes a qualitative assessment of the severity of involvement in each region within the maxilla. There are three possible levels of severity for each of the four GAND parameters evaluated, ranging from less to more affected. As a supplement to the “G” component of the GAND classification and measuring the physical dimensions of the cleft, we use the Chelsea scale to describe the patterns of bone bridging of the interdental septum associated with the cleft.16 The Chelsea system categorizes the volume and position of the interseptal bony bridge into one of six categories: A to F (Figure 5). Of these, A, B, and C are considered to be acceptable, whereas D, E, and F are less than satisfactory.
We describe the overall cleft defect and affected adjacent tissues on appropriately formatted CBCT images according to the gap, arch, nasal floor, and dental (GAND) classification15 (Table 2, and Figure 2 through Figure 4). This includes a qualitative assessment of the severity of involvement in each region within the maxilla. There are three possible levels of severity for each of the four GAND parameters evaluated, ranging from less to more affected. As a supplement to the “G” component of the GAND classification and measuring the physical dimensions of the cleft, we use the Chelsea scale to describe the patterns of bone bridging of the interdental septum associated with the cleft.16 The Chelsea system categorizes the volume and position of the interseptal bony bridge into one of six categories: A to F (Figure 5). Of these, A, B, and C are considered to be acceptable, whereas D, E, and F are less than satisfactory.
 Because the treatment of patients with CLP is multidisciplinary, these individuals often have diagnostic records obtained from multiple clinicians from different disciplines. This can include not only cone-beam imaging data, but also extra- and intraoral optical scans obtained using different systems. Distribution of these data between healthcare providers is essential for timely and appropriate treatment. Sharing and immediate access to data can be facilitated using a secure Health Insurance Portability and Accountability Act (HIPAA)-compliant picture archiving and communication system (PACS) server that houses all records for the team’s immediate access.
Because the treatment of patients with CLP is multidisciplinary, these individuals often have diagnostic records obtained from multiple clinicians from different disciplines. This can include not only cone-beam imaging data, but also extra- and intraoral optical scans obtained using different systems. Distribution of these data between healthcare providers is essential for timely and appropriate treatment. Sharing and immediate access to data can be facilitated using a secure Health Insurance Portability and Accountability Act (HIPAA)-compliant picture archiving and communication system (PACS) server that houses all records for the team’s immediate access.
At our institution, we internally distribute records using a medical PACS that incorporates a dental reformatting module. Because our referral base has expanded to include many more referrals from external clinicians, we are investigating the use of a subscription-based cloud PACS system. This would eliminate redundant imaging, providing a cost-efficient, central, HIPAA-compliant storage and external on-demand distribution and visualization portal.
Conclusion
In closing, treating patients with CLP is a complex and multidisciplinary task requiring periodic age-based radiographic imaging. Protocols for imaging should be based on specific diagnostic criteria designed to support optimal treatment planning and case management. These protocols should use the nominal number of radiographic examinations required, and all imaging should use exposure settings that produce diagnostically acceptable images at the lowest possible radiation dose to the patient.
References
- De Mulder D, Cadenas de Llano-Pérula M, Willems G, et al. An optimized imaging protocol for orofacial cleft patients. Clin Exp Dent Res. 2018;4:152–157.
- Kosowski TR, Weathers WM, Wolfswinkel EM, Ridgway EB. Cleft palate. Semin Plast Surg. 2012;26:164–169.
- Mangold E, Ludwig KU, Nöthen MM. Breakthroughs in the genetics of orofacial clefting. Trends Mol Med. 2011;17:725–733.
- Tessier P. Anatomical classification facial, cranio-facial and latero-facial cleftsJ J Maxillofac Surg. 1976;4:69–92.
- Kernahan DA. The striped Y a symbolic classification for cleft lip and palate. Plast Reconstr Surg. 1971;47:469–470.
- Parker SE, Mai CT, Canfield MA, et al. Updated national birth prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A Clin Mol Teratol. 2010;88:1008–1016.
- Abramson ZR, Peacock ZS, Cohen HL, Choudhri AF. Radiology of cleft lip and palate: imaging for the prenatal period and throughout life. Radiographics. 2015;35:2053–2063.
- Kuijpers MA, Chiu YT, Nada RM, Carels CE, FudaleJ PS. Three-dimensional imaging methods for quantitative analysis of facial soft tissues and skeletal morphology in patients with orofacial clefts: A systematic review. PLoS One. 2014;9:6–9.
- Jacobs R, Pauwels R, Scarfe WC, et al. Pediatric cleft palate patients show a 3- to 5-fold increase in cumulative radiation exposure from dental radiology compared with an age- and gender-matched population: a retrospective cohort study. Clin Oral Investig. 2018;22:1783–1793.
- Scarfe WC, Farman AG. What is cone-beam CT and how does it work? Dent Clin North Am. 2008;52:707–730.
- Jaju PP, Jaju SP. Cone-beam computed tomography: time to move from ALARA to ALADA. Imaging Sci Dent. 2015;45:263–265.
- American Academy of Pediatric Dentistry. Prescribing Dental Radiographs for Infants, Children, Adolescents, and Individuals With Special Health Care Needs. Available at: http/://www.aapd.org/globalassets/media/polici_s_guidelines/bp_ radiographs.pdf. Accessed April 13, 2020.
- Oenning AC, Jacobs R, Pauwels R, Stratis A, Hedesiu M, Salmon B. Cone-beam CT in paediatric dentistry: DIMITRA project position statement. Pediatr Radiol. 2018;48:308–316.
- De Mulder D, De Llano-Pérula MC, Jacobs R, Jacobs R, Verdonck A, Willems G. Three-dimensional radiological evaluation of secondary alveolar bone grafting in cleft lip and palate patients: a systematic review. Dentomaxillofacial Radiol. 2019;48:1–13.
- Barbosa GL, Emodi O, Pretti H, et al. GAND classification and volumetric assessment of unilateral cleft lip and palate malformations using cone beam computed tomography. Int J Oral Maxillofac Surg. 2016;45:1333–1340.
- Witherow H, Cox S, Jones E, Carr R, Waterhouse N. A new scale to assess radiographic success of secondary alveolar bone grafts. Cleft Palate Craniofacial J. 2002;39:255–260.
From Dimensions of Dental Hygiene. February 2022; 20(2):22-25.

