 SELVANEGRA/ISTOCK/GETTY IMAGES PLUS
SELVANEGRA/ISTOCK/GETTY IMAGES PLUS
Host Modulation and the Inflammatory Response
Host modulation therapy may be effective in controlling the immune response responsible for periodontal diseases.
This course was published in the March 2020 issue and expires March 2023. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Discuss the relationship between inflammation and the host response.
- Identify host modulation therapies used to treat chronic inflammatory diseases.
- Explain how host modulation therapies may be effective in addressing periodontal diseases.
Periodontal diseases are related to an immunoinflammatory response to various types of oral biofilm. They affect approximately 42% of United States adults.1 Chronic periodontitis is characterized by alveolar bone loss, attachment loss, and apical migration of the junctional epithelium. Periodontal diseases result from dysbiosis: an imbalance between different organisms present in the natural microbiota of the subgingival microenvironment. Periodontal destruction can be attributed to the interaction of two main players: periodontally pathogenic biofilm and the host-mediated immunoinflammatory response to that biofilm.1 Current treatment guidelines for periodontitis include the physical removal of plaque biofilm and calculus by scaling and root planing.² While this treatment aims to eliminate the bacterial insult, scaling and root planing does not address the host response element of periodontal diseases.³
Inflammation is the body’s initial response to various types of injury via physical, bacterial, or chemical means, and is characterized by an increase in temperature, erythema, edema, pain, and loss of function.4,5 During an acute inflammatory response, a series of events occurs including increased vessel permeability, emigration of leukocytes to the site of injury, and activation of macrophages, which ingest foreign substances, toxins, and debris.4,6 If the acute injury is eliminated, tissue repair begins in an effort to return to its original state.4 If inflammation persists due to continued insult or an exaggerated immune response, tissue damage continues.1,6 The chronic inflammatory response involves various cells of the acquired immune system: macrophages, lymphocytes, and plasma cells and their products.4,5 The development of periodontal diseases is initiated by a dysbiotic bacterial plaque insult and tissue destruction is propagated by the host immune response to the biofilm. This response differs among individuals, which may explain why some patients have low levels of bacterial plaque deposits and excellent oral hygiene, but still experience chronic inflammation and bone loss.1,4,7–11
Host modulation therapies address the host immune response to an antigen or antigens.5 They aim to disrupt immunoinflammatory pathways within the host’s response to those pathogens that perpetuate an inflammatory response.12 Several different pathways can initiate an inflammatory response, including B-cells, T-cells, and macrophages. They produce pro-inflammatory cytokines or signaling proteins to coordinate the immune response, resulting in either pro- or anti-inflammatory results. Variations in disease severity may be due to an increased number of B cells or plasma cells in response to a pathogen and/or an overproduction of their byproducts, such as matrix metalloproteinases (MMPs), cytokines, prostaglandins (PG), and other inflammatory mediators.1,7,12,13
Host modulation therapies are also used to treat other chronic inflammatory diseases, such as rheumatoid arthritis (RA), Crohn disease, and psoriasis. RA is similar to periodontal diseases in regards to the bone loss and collagen destruction caused by the host inflammatory response.3,6,13,14 Treatments for RA include non-steroidal anti-inflammatory drugs (NSAIDs), steroids, and disease modifying anti-rheumatic drugs (eg, methotrexates, immunosuppressants, and cytokine-targeting therapies). However, side effects such as liver damage, nausea, hair loss, rashes, and lung infections typically preclude their use for periodontal disease treatment.5,15 Current cytokine-modulation therapies target cytokines common in both RA and periodontitis. Anakinra, a drug approved for treating RA, is a recombinant, modified version of the interleukin-1 receptor antagonist that competes with the proinflammatory cytokine IL-1 for receptors, therefore preventing or reducing inflammation. Additionally, infliximab is a monoclonal antibody drug used to neutralize tumor necrosis factor alpha (TNF-α), a pro-inflammatory cytokine implicated in both RA and periodontal diseases.3 These treatments are used as adjuncts to traditional immunosuppressive therapy and have limited effectiveness in reducing symptoms of RA as monotherapies. However, with continued research, these pathways may provide future treatment options for both RA and periodontal diseases.2,14
HOST MODULATION THERAPY OPTIONS FOR PERIODONTAL DISEASES
A variety of potential host-modulation therapies are available to treat periodontal diseases, including NSAIDs, subantimicrobial-dose doxycycline (SDD), bisphosphonates, and cytokine inhibitors (Table 1). However, most of these therapies are not yet proven or approved treatments for periodontitis. NSAIDs regulate the host response by decreasing PGs, pro-inflammatory hormones that demonstrate local upregulation in response to lipopolysaccharides (LPS) present in plaque biofilm. NSAIDs are known to be direct inhibitors of cyclooxygenase, an enzyme involved in tissue breakdown via the production of arachidonic acid metabolites that lead to activation of osteoclasts and MMPs.4,7,8,16 While NSAIDs are proven to decrease PG synthesis, the side effects of long-term use have prevented it from being incorporated into regular periodontal treatment.17 A recent systematic review revealed clinical benefits for the use of NSAIDs in conjunction with nonsurgical periodontal treatment in reducing the rate of gingival inflammation and alveolar bone resorption. However, these benefits did not extend to the improvement of probing depth or clinical attachment level.9 Unwanted side effects may also be reduced by the use of novel NSAID therapies, NSAID dentifrices, and/or lower dose NSAIDs.17 Geisinger et al18 concluded that the addition of low‐dose aspirin therapy in patients with chronic periodontitis undergoing nonsurgical periodontal therapy was beneficial. Aspirin reduces inflammatory mediators and triggers production of resolvin D3, an inflammation resolving mediator derived from the metabolism of omega-3 fatty acids, by modifying cells’ production of cyclo-oxygenase type 2, another inflammation-inducing PGs. Additional studies on the use of adjunctive aspirin therapy in the treatment of periodontal diseases are needed.
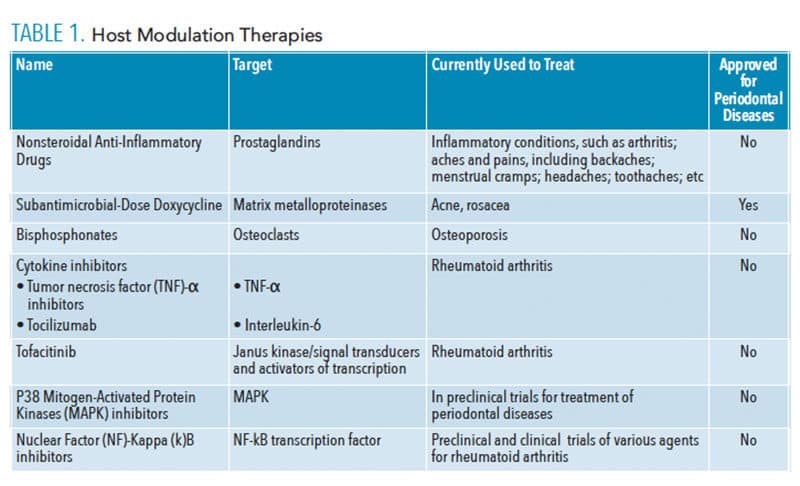 Administration of SDD helps to reduce MMP activity, thus preventing collagen destruction in periodontal diseases.7,19 Tetracyclines have been used as antimicrobials in higher doses to reduce bacterial load but also promote antibiotic resistance at these dosages. In the treatment of periodontal diseases, a subantimicrobial dose reduces inflammation via pathways unrelated to antimicrobial activity, thus they do not result in antibiotic resistance.19,20 In health, MMPs are secreted by fibroblasts, keratinocytes, macrophages, polymorphonuclear leukocytes, and endothelial cells, and support normal connective tissue and bone remodeling. In disease, overproduction of MMPs can lead to collagen destruction and bone loss.7,20 Tetracyclines have been shown to increase levels of transforming growth factor β1 (TGF-β) in the gingival crevicular fluid, which is an important mediator in wound healing that stimulates fibroblast proliferation and also inhibits MMPs.7,20,21 SDD treatment has few side effects, can help reduce inflammation, improves treatment outcomes, and is recommended by the American Dental Association as an adjunct for the nonsurgical treatment of moderate to severe chronic periodontitis in conjunction with scaling and root planing.2,19
Administration of SDD helps to reduce MMP activity, thus preventing collagen destruction in periodontal diseases.7,19 Tetracyclines have been used as antimicrobials in higher doses to reduce bacterial load but also promote antibiotic resistance at these dosages. In the treatment of periodontal diseases, a subantimicrobial dose reduces inflammation via pathways unrelated to antimicrobial activity, thus they do not result in antibiotic resistance.19,20 In health, MMPs are secreted by fibroblasts, keratinocytes, macrophages, polymorphonuclear leukocytes, and endothelial cells, and support normal connective tissue and bone remodeling. In disease, overproduction of MMPs can lead to collagen destruction and bone loss.7,20 Tetracyclines have been shown to increase levels of transforming growth factor β1 (TGF-β) in the gingival crevicular fluid, which is an important mediator in wound healing that stimulates fibroblast proliferation and also inhibits MMPs.7,20,21 SDD treatment has few side effects, can help reduce inflammation, improves treatment outcomes, and is recommended by the American Dental Association as an adjunct for the nonsurgical treatment of moderate to severe chronic periodontitis in conjunction with scaling and root planing.2,19
Bisphosphonates are bone-sparing agents that modify the host response by disrupting osteoclastic activity, which inhibits bone resorption. Bone sparing drugs are used to treat metabolic bone diseases but are not recommended for the treatment of periodontal diseases. In addition to disrupting osteoclastic activity, bisphosphonates also induce osteoclastic apoptosis (programmed cell death), and may also inhibit MMP activity or provide anti-collagenase properties.7,8,16,17 Lane et al22 found that bisphosphonates provided benefits in clinical attachment levels, probing depths, and bleeding on probing. Despite this promising study, bisphosphonates are not without side effects, including changes in white blood cell production and, rarely, medication-related osteonecrosis of the jaw. They are currently only approved for systemic bone loss by the US Food and Drug Administration (FDA). However, future incarnations of bisphosphonates may be used to treat periodontal bone loss.7,16,17
Host modulation therapies that target specific cytokine pathways are used as second-line therapies for RA and may be helpful in periodontitis treatment.5,23 Microbes produce a variety of pathogenic substances including glycoproteins, lipoproteins, lipoteichoic acids, viral ribonucleic acids (RNA), and lipopolysaccharides. These substances are recognized by a family of receptors known as toll-like receptors that are membrane-spanning proteins found on macrophages, monocytes, and dendritic cells. They activate a cascade of intracellular signaling molecules, including mitogen activated protein kinases (MAPKs) and transcription factors such as nuclear factor-kB (NF-kB). These cell signaling molecules and transcription factors ultimately lead to the production of pro-inflammatory cytokines.24–27 Early research focused on cytokine inhibition by the use of antibodies or fusion proteins to TNF-α.15,28 Several of these TNF-α inhibitors were approved by the FDA for use in RA due to their ability to slightly decrease bone resorption and increase bone formation.10,23 However, side effects include increased rates of infection and bone marrow suppression.5,15,23 A systematic review of TNF-α inhibitors used in patients with RA and its effect on periodontitis revealed that those taking TNF-α inhibitors may have reduced progression of periodontal disease and reduced periodontal TNF-α levels.15 This topic warrants further research.
The direct targeting of intracellular cell signaling proteins involved in the production of pro-inflammatory molecules is another approach. MAPKs are a family of intracellular-signaling molecules activated by cytokines or LPS, and result in upregulation of pro-inflammatory cytokines, MMPs, and PGs.28 Phase II clinical trials of P38 MAPK inhibitors have shown promising results in the short term.29 Another potential avenue for intracellular host modulation is the Janus kinase/signal transducers and activators of transcription (JAK/STAT) signal transduction pathway stimulated by cytokines and growth factors. Dysregulation of the JAK/STAT pathway can lead to chronic inflammatory diseases and blood cancers.8,30 One of the challenges surrounding the study of cell-signaling protein inhibitors is that the cascade of proteins involved in cell signaling pathways are often involved in cross-talk with each other, thus activation of one pathway can lead to simultaneous activation of another pathway.31
EMERGING TREATMENTS THAT MAY TARGET PERIODONTITIS
The host modulatory system can be targeted via transcription factors activated by the inflammatory response. Transcription factors are proteins that bind to deoxyribonucleic acid- or DNA-promoters, and regulate gene activation.32 NF-kB is a family of transcription factors activated by pro-inflammatory cytokines and helps to produce additional pro-inflammatory cytokines.33 This leads to chronic inflammation. Current research has led to phase II clinical trials on several different drugs that target the NF-kB pathway in patients with RA.34 A study of patients with healthy vs periodontally involved tissues showed an upregulation of NF-kB in diseased states, suggesting that therapeutic targeting of NF-kB may help improve the immune-mediated component of periodontal diseases.34,35 Several other proteins within the cell signaling pathway may yield future treatment options but research is currently limited.5,7,8
The cellular/molecular mechanisms underlying the anti-inflammatory effect of polyunsaturated fatty acids (PUFA) are unknown, but their use as host modulatory agents is promising. The predominant PUFAs are the n-6 series (found in soy, corn and sunflower oils) and n-3 series (found mainly in oily fish, although there are plant sources), also known as omega-3 fatty acids. The three types of omega−3 fatty acids are α-linolenic acid, eicosapentaenoic acid, and docosahexaenoic acid. Consuming fish-derived omega−3 fatty acids lowers blood markers of inflammation.36 In a study of 80 subjects, researchers examined the effectiveness of combining omega-3 fatty acids and aspirin to treat periodontitis. All subjects received scaling and root planing, but the subjects in the fish oil/aspirin group received daily supplementation of fish oil and 81 mg of aspirin. After 3 months and 6 months, subjects in the fish oil/aspirin group exhibited reduction in probing depths, attachment gain, and reductions of salivary biomarkers of bone resorption.37
Statins lower cholesterol in the blood by inhibiting the synthesis of mevalonate, the precursor of cholesterol. Statins have also demonstrated anti-inflammatory and immunomodulatory effects (inhibit tissue-degrading enzymes) and interfere with osteoclasts. Currently, statins are used to treat osteoporosis and have been used to enhance bone formation and reduce inflammation in dentistry.38 Statins seem to increase production of mRNA of osteoprotegerin by osteoblasts, thereby reducing bone resorption. A recent meta-analysis showed that application of locally applied statins as an adjunct to mechanical periodontal treatment produced larger improvements in terms of periodontal depth reduction and gain of clinical attachment.39 However, little research is available on the use of statins as an adjunct to conventional periodontal therapy and further research is needed.
Teriparatide, a form of parathyroid hormone (PTH), has been approved by the FDA for the treatment of osteoporosis. PTH increases serum calcium, and chronically elevated PTH will deplete bone stores. However, intermittent exposure to PTH will activate osteoblasts more than osteoclasts.40 Bashutski et al41 conducted a trial of 40 adults with chronic periodontitis who underwent periodontal surgery and daily injections of teriparatide. The treatment group experienced greater radiographic resolution of periodontal bone defects compared to the placebo group, with accelerated osseous wound healing in the oral cavity. Teriparatide joins the growing list of agents used to encourage tissue and organ regeneration.42
CONCLUSION
While SDD is currently the only FDA-approved host modulation therapy for periodontitis, the success of using host modulation therapy in the treatment of other chronic inflammatory diseases proves that host modulation may be effective in controlling the immune response responsible for periodontal diseases.12,17 Targeting the inflammatory pathways that lead to dysbiosis and chronic inflammation is ideal to reduce tissue destruction related to host immunoinflammatory activity. Cytokine pathways may provide future drug targets that will modify the host’s immune response in periodontal diseases, but thus far have only been studied with other autoimmune diseases. Data regarding outcomes for periodontitis are still largely unknown. More research is needed on the effects of host modulation therapies specifically on periodontal diseases.5 Currently, the side effects associated with host modulation medications for RA preclude their safe use in patients exclusively with periodontal diseases. In the future, additional research may yield safer drugs, dosages, and/or delivery methods that can also treat periodontitis.
REFERENCES
- Eke PI, Thornton-Evans GO, Wei L, Borgnakke WS, Dye BA, Genco RJ. Periodontitis in US adults: National health and nutrition examination survey 2009-2014. J Am Dent Assoc. 2018;149: 576–588.
- Smiley C Tracy SL, Abt E, et al. Evidence-based clinical practice guideline on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J Am Dent Assoc. 2015;146:525–535.
- Dinarello C. Therapeutic strategies to reduce IL-1 activity in treating local and systemic inflammation. Curr Opin Pharmacol. 2004;4:378–385.
- Hasturk H, Kantarci A. Activation and resolution of periodontal inflammation and its systemic impact. Periodontol 2000. 2015;69:255–273.
- Preshaw PM. Host modulation therapy with anti-inflammatory agents. Periodontol 2000. 2017;76:131–149.
- Nibali L, Fedele S, Donos N, D’Aiuto F. The role of interleukin-6 in oral diseases. Dimensions of Dental Hygiene. 2013;11(1):28–34.
- Elavarasu S, Sekar S, Murugan T. Host modulation by therapeutic agents. J Pharm Bioallied Sci. 2012;4(Suppl 2):S256–S259.
- Souza JACD, Junior CR, Garlet GP, Nogueira AVB, Cirelli JA. Modulation of host cell signaling pathways as a therapeutic approach in periodontal disease. J Appl Oral Sci. 2012;20:128–138.
- Kang DY, Cho IW, Shin HS, Ahn HS, Kim HJ, Park JC. Effects of host modulation by nonsteroidal anti-inflammatory drugs on periodontal disease: a systematic review and meta-analysis. J Dent Rehabil Appl Sci. 2017;33:7–18.
- Silva N, Abusleme L, Bravo D, et al. Host response mechanisms in periodontal diseases. J Appl Oral Sci. 2015;23:329–355.
- Baker PJ. The role of immune responses in bone loss during periodontal disease. Microbes Infect. 2000;2:1181–1192.
- Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov. 2004;3:401–416.
- Zhang J-M, An J. Cytokines, inflammation and pain. Int Anesthesiol Clin. 2007;45:27–37.
- Koziel J, Mydel P, Potempa J. The link between periodontal disease and rheumatoid arthritis: an updated review. Curr Rheumatol Rep. 2014;16:3.
- Han JY, Reynolds MA. Effect of anti-rheumatic agents on periodontal parameters and biomarkers of inflammation: a systematic review and meta-analysis. J Periodontal Implant Sci. 2012;42:3–12.
- Shinwari MS, Tanwir F, Hyder PR, Bin Saeed MH. Host modulation therapeutics in periodontics: role as an adjunctive periodontal therapy. J Coll Physicians Surg Pak. 2014;24:676–684.
- Bhatt A, Govila V, Sharma M. Host modulatory agents in periodontics: a step towards the future. J Int Clin Dent Res Org. 2015;7:130.
- Geisinger M, Holmes C, Vassilopoulos V, Geurs N, Reddy M. Adjunctive treatment of chronic periodontitis with systemic low‐dose aspirin therapy. Clinical Advances in Periodontics. 2012; 2:195–199.
- Golub LM, Mcnamara TF, Ryan ME, et al. Adjunctive treatment with subantimicrobial doses of doxycycline: effects on gingival fluid collagenase activity and attachment loss in adult periodontitis. J Clin Periodontol. 2001;28:146–156.
- Walker SG, Golub LM. Host modulation therapy for periodontal disease: subantimicrobial-dose doxycycline, medical as well as dental benefits. Available at: oralhealthgroup.com/features/host-modulation-therapy-for-periodontal-disease-subantimicrobial-dose-doxycycline-medical-as-well-as/. Accessed February 23, 2020.
- Gürkan A, Emingil G, Çınarcık S, Berdeli A. Post-treatment effects of subantimicrobial dose doxycycline on clinical parameters and gingival crevicular fluid transforming growth factor-β1 in severe, generalized chronic periodontitis. Int J Dent Hyg. 2008;6:84–92.
- Lane N, Armitage GC, Loomer P, et al. Bisphosphonate therapy improves the outcome of conventional periodontal treatment: results of a 12-month, randomized, placebo-controlled study. J Periodontol. 2005;76:1113–1122.
- Morand DN, Davideau JL, Clauss F, Jessel N, Tenenbaum H, Huck O. Cytokines during periodontal wound healing: potential application for new therapeutic approach. Oral Dis. 2017;23:300–311.
- Brightbill HD, Modlin RL. Toll-like receptors: molecular mechanisms of the mammalian immune response. Immunol. 2000;101:1–10.
- Vasselon T, Hanlon WA, Wright SD, Detmers PA. Toll‐like receptor 2 (TLR2) mediates activation of stress‐activated MAP kinase p38. J Leukoc Biol. 2002;71:503–510.
- Li Q, Valerio MS, Kirkwood KL. MAPK usage in periodontal disease progression. J Signal Transduct. 2012;2012:308943.
- Jiménez-Dalmaroni MJ, Gerswhin ME, Adamopoulos IE. The critical role of toll-like receptors—from microbial recognition to autoimmunity: a comprehensive review. Autoimmun Rev. 2016;15:1–8.
- Kobayashi T, Ito S, Kobayashi D, et al. Interleukin‐6 receptor inhibitor tocilizumab ameliorates periodontal inflammation in patients with rheumatoid arthritis and periodontitis as well as tumor necrosis factor inhibitors. Clin Exp Den Res. 2015;1:63–73.
- Kirkwood KL, Cirelli JA, Rogers JE, Giannobile WV. Novel host response therapeutic approaches to treat periodontal diseases. Periodontol 2000. 2007;43:294–315.
- Rawlings JS. The JAK/STAT signaling pathway. J Cell Sci. 2004;117:1281–1283.
- Damjanov N, Kauffman RS, Spencer‐Green GT. Efficacy, pharmacodynamics, and safety of VX‐702, a novel p38 MAPK inhibitor, in rheumatoid arthritis: results of two randomized, double‐blind, placebo‐controlled clinical studies. Arthritis & Rheum. 2009;60:1232–1241.
- Phillips T, Hoopes L. Transcription factors and transcriptional control in eukaryotic cells. Available at: nature.com/scitable/topicpage/transcription-factors-and-transcriptional-control-in-eukaryotic-1046. Accessed February 23, 2020.
- Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2.
- Noort AR, Tak PP, Tas SW. Non-canonical NF-κB signaling in rheumatoid arthritis: Dr. Jekyll and Mr Hyde? Arthritis Res Ther. 2015;17:15.
- Ambili R, Santhi WS, Janam P, et al. Expression of activated transcription factor nuclear factor-κB in periodontally diseased tissues. J Periodontol. 2005;76:1148–1153.
- Chee B, Parkl B, Fitzsimmons T, Coates A, Bartold P. Omega-3 fatty acids as an adjunct for periodontal therapy—a review. Clin Oral Invest. 2016;20:879–894.
- El-Sharkawy H, Aboelsaad N, Eliwa M, et al. Adjunctive treatment of chronic periodontitis with daily dietary supplementation with omega‐3 fatty acids and low‐dose aspirin. J Periodontol. 2010; 81:1635–1643.
- Estanislau I, Terceiro IR, Lisboa MR, et al. Pleiotropic effects of statins on the treatment of chronic periodontitis–a systematic review. Br J Clin Pharmacol. 2015;79:877–885.
- Bertl K, Parllaku A, Pandis N, Buhlin K, Klinge B, Stavropoulos A. The effect of local and systemic statin use as an adjunct to non-surgical and surgical periodontal therapy—A systematic review and meta-analysis. J Dent. 2017;67:18–28.
- Bodenner D, Redman C, Riggs A. Teriparatide in the management of osteoporosis. Clin Interv Aging. 2007;2:499–507.
- Bashutski JD, Eber RM, Kinney JS, et al. Teriparatide and osseous regeneration in the oral cavity. N Engl J Med. 2010;363:2396–2405.
- Larsson L, Decker AM, Nibali L, Pilipchuk SP, Berglundh T, Giannobile WV. Regenerative Medicine for Periodontal and Peri-implant Diseases. J Dent Res. 2016;95:255–266.
From Dimensions of Dental Hygiene. March 2020;18(3):26–28,31.



