HIV Testing in the Dental Setting
Oral health professionals are well positioned to identify the early stages of human immunodeficiency virus infection.
This course was published in the October 2017 issue and expires October 2020. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Explain the science and incidence of human immunodeficiency virus (HIV) infection, and the fundamentals of transmission.
- List common oral complications of HIV and acquired immunodeficiency syndrome.
- Describe the dental team’s role in screening and the basics of rapid HIV testing.
The oral cavity and salivary diagnostics may aid in the early discovery of systemic disease. Modern diagnostic tools can detect the presence of subclinical disease, and oral health professionals have the opportunity to provide patients with diagnostic screening for human immunodeficiency virus (HIV). Test results from the dental office indicate reactivity and are not diagnostic; if a patient is determined to be reactive, confirmatory tests are required.
Associated with acquired immunodeficiency syndrome (AIDS), HIV has been identified as causative factor for more than 30 years.1 The incidence of HIV infection has continued to rise, and is estimated at 50,000 new cases per year in the United States.2 According to the US Centers for Disease Control and Prevention (CDC), nearly 13% of the more than 1.2 million infected Americans do not know they are HIV positive.3 Offering screening that can help detect HIV infection status may facilitate early medical intervention.
As a retrovirus, HIV carries its genetic material in the form of ribonucleic acid (RNA), rather than deoxyribonucleic acid (DNA). In order to replicate, it must find a host cell. It does so by seeking the T lymphocytes (derived from the thymus) of the immune system. T lymphocytes are able to produce and express various glycoproteins. One of these proteins is CD4, and once the CD4 protein is expressed and attached to the surface of the T lymphocyte, it is referred to as a CD4+ T helper cell—or, more commonly, a CD4 cell. When HIV is introduced into the body, it locates and enters the CD4 cells. The virus then rapidly replicates in the host CD4 cell.
The CD4 cell is an important factor in regulation of the immune response and antibody production. During the first few weeks of infection, the immune system’s response to this invasion is to increase the number of CD4 cells. Once the virus completes its replication, it destroys the CD4 cell. As the virus continues to multiply, it rapidly invades and destroys more CD4 cells than the immune system can produce, leading to a drop in CD4 counts. In the late stages of infection, the cell count drops to a level that compromises the immune system’s ability to fight opportunistic diseases. When the CD4 cells are depleted below 200/microliter, the individual is diagnosed with AIDS.4 An individual may be considered to have AIDS if one or more opportunistic infections associated with AIDS is exhibited.
The virus requires various enzymes to continue its life cycle. Treatment for HIV involves interruption of the HIV life cycle. Antiretroviral therapy (ART) works by inhibiting these enzymes.5 In the cytoplasm, viral RNA is converted to DNA by the enzyme viral reverse transcriptase. At this point, the viral DNA enters the nucleus, where it is combined with the human DNA by the enzyme viral integrase. Finally, new viral particles containing mRNA are produced by the enzyme viral protease. These new viral particles, referred to as “copies,” are now able to infect additional CD4 cells.6 Various drugs can halt this process and, when indicated, multiple drug cocktails are referred to as highly active antiretroviral therapy (HAART).7 HAART treatment typically includes a combination of three or more drugs. Each drug is specific to an enzyme needed for the virus’ replication.

TRANSMISSION
Multiple factors contribute to the transmission of HIV. For the purposes of this article, evaluating the risk of an individual contracting HIV from an infected partner will focus on two risk factors, including type of exposure and the viral load at contact. These exposure types include sexual behavior exposure and parenteral exposure.
Risk based on exposure from sexual behavior is predicated on sexual practices, yet the sexual practice with the greatest risk accounts for a risk probability of 138 in 10,000 exposures.7 This is rather low compared to risk based on parenteral exposure. The highest probability for transference of the virus is associated with behaviors in the parenteral group. Blood transfusion, for example, carries the greatest risk. But even needle-sharing (such as in drug use) and percutaneous needlestick carry a significantly higher risk than many subcategories of risk based on sexual behavior.8
An individual’s HIV infection status is initially determined by his or her CD4 cell count and viral load,9 which is the amount of HIV present in the blood. Viral load is a measure of HIV genetic material (RNA) in terms of the number of copies of the virus, and is also used to determine the effectiveness of ART.9
In terms of sexual practices, the risk of exposure to HIV varies. Transmission risk decreases when the viral load is low and increases when the viral load is high.10 Laboratories measure viral load in terms of the number of copies of the virus per milliliter of plasma. The use of ART can drop the viral load to an optimal level of between 20 copies/ml to 75 copies/ml,11 and it can also reduce it to very low or undetectable levels. Although individuals with undetectable or very low viral levels are unlikely to transmit HIV, transmission is still possible.10
Viral load varies depending on the stage of HIV infection. In the early or late stages of infection, viral load is high.4 If exposure occurs at a stage that is associated with a lower viral load—such as when a patient is on medication and the CD4 count is indicative of a stronger immune system—the risk potential of any given behavioral factor is reduced. If the medications are not taken as directed, the viral load can be expected to increase and the risk of transmission rises accordingly.

CLINICAL PRESENTATION OF INFECTION
As part of a national response to the AIDS epidemic, the White House developed the National HIV/AIDS Strategy.12 These efforts have advanced the care provided to HIV-infected Americans so that health professionals now see fewer oral manifestations in patients infected with HIV. The introduction of HAART has lowered the prevalence of oral manifestations of HIV infection by 30%.13However, oral manifestations do sometimes present, and can be fungal, viral, bacterial or neoplastic in nature.
Common oral manifestations of HIV include:
- Erythematous candidiasis often presents on the dorsum of the tongue, but is also seen on the hard palate or soft palate. It is red and flat in appearance (Figure 1).
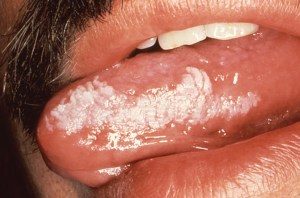
- Pseudomembranous candidiasis presents on the tongue and oral mucosa. It appears as white plaques and leaves a red or bleeding surface after being wiped off (Figure 2).
- Oral hairy leukoplakia is seen on the lateral borders of the tongue, and has a white corrugated appearance that cannot be wiped off (Figure 3).
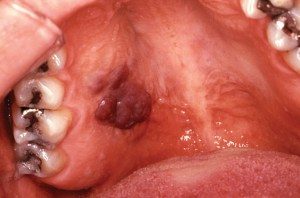
- Kaposi’s sarcoma appears as a macular or nodular lesion. It may or may not be ulcerated. These lesions can be red or even purple, and older lesions are darker. (Figure 4).
The use of ART has decreased the incidence of some oral manifestations of HIV.14 Retroviral therapy interrupts the HIV life cycle, thereby allowing for the presence of more CD4 cells.
TESTING IN THE DENTAL SETTING
Arguments have been made for and against HIV testing in the dental setting. One contention is that the nature of this test is beyond the scope of dental practice. Other arguments include its impact on productivity and patient perception. Conversely, screening by dental teams may help identify HIV infection, possibly in its early stages, thus facilitating referral for medical intervention that can prolong a patient’s life and prevent the spread of the virus. Recommendations from the CDC emphasize the value of early detection. A more empirical argument in favor of testing in the dental setting is that most patients are more likely to regularly visit their dentist than physician.15
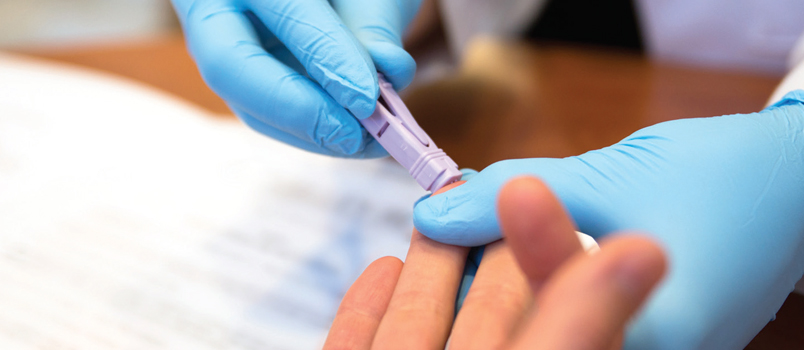
Rapid HIV screening can be completed in the dental office; salivary testing, for example, requires approximately 20 minutes to 30 minutes. Patients should receive a clear explanation that this is a screening tool, and the results are only preliminary. Any patient who tests preliminarily reactive should be linked to the next step in care. The provider should facilitate an appointment with a medical HIV specialist. This potentiality should be included in the informed consent document. If an appointment cannot be made at time of service, the oral health care provider must follow up within 48 hours. In this respect, having interprofessional connections that can expedite follow-up care with local HIV specialists and psychological counselors is important. Office protocol must include procedures for ensuring the proper professional is contacted and that a follow-up appointment is made.
A positive preliminary test result is likely to be disturbing, and patients may display a wide range of emotions. Dental teams who engage in HIV screening should receive specialized training from mental health experts on how to best interact with these patients. Having multiple referral resources for psychological counseling immediately available to the patient is recommended. Patients should also be informed that if a definitive test is HIV positive, this is a manageable chronic condition. Being HIV positive does not automatically mean the individual has AIDS.16 It is advisable to emphasize the efficacy of ART and inform the patient that, with early treatment, the virus can be controlled for years.17
It is not unusual for an early HIV infection to show no symptoms, and an infected individual may not seek medical care until the condition has reached an advanced stage. Because oral health professionals are often a first point of contact in the health care delivery system, HIV screening in dental practice can help detect infection. If a patient is diagnosed at an early stage, medical intervention is more effective, as early treatment reduces the viral load.
RAPID TESTING
Testing for HIV can be done in three ways. A test can detect antibodies, antigens (together with antibodies), or the presence of nucleotides.18 Nucleotides are the molecular subunits of DNA and RNA, and for the latter include the bases adenine, guanine, uracil, and cytosine.19 Testing for these elements is complex and best accomplished by a diagnostic lab. Screening performed in dental settings tests for antibodies. This test has an exposure window of up to 3 months.18 It may not yield results for exposures occurring within the past 3 months because antibodies to HIV are not developed immediately. It may take up to 3 months before blood will show evidence of antibodies to the HIV antigen.18 If a shorter exposure window is desired, the individual should see a medical provider who can perform a nucleotide test, which has an exposure window of 9 days to 14 days.20
Before conducting HIV testing in the dental setting, the office should formulate a written protocol. Each state may differ in its regulations for testing and follow-up procedures. The office protocol must be consistent with state regulations.
The Clinical Laboratory Improvement Amendments of 1988 (CLIA) include federal standards that regulate all US facilities that test human specimens. Under CLIA, any facility that examines such specimens must register with the federal Centers for Medicare and Medicaid Services and obtain a CLIA certification. However, the US Food and Drug Administration (FDA) has approved various rapid HIV tests as “waived tests” under CLIA. Well suited for dental settings, waived tests must use unprocessed blood or oral fluid, be easy to use, and have little risk of an incorrect result.21 Rapid HIV screening in dental settings using a CLIA-waived test requires a CLIA Certificate of Waiver.21 In addition, most states require that dental offices have a protocol for HIV testing that addresses testing procedures, staff training, temperature monitoring, quality control, confidentiality, linkage to follow-up care, and tracking and recording.
The US Preventive Services Task Force recommends that pregnant women and all individuals between the ages of 15 and 65 be screened for HIV.22 Patient consent is a consideration. In 2006, the CDC recommended that patients be informed verbally or in writing that HIV testing is part of the general treatment.23 Having received this information, the patient can refuse the test. According to this recommendation, a specific HIV testing consent is not required.23 This is a recommendation. Each state has the authority to make its own requirements regarding what defines informed consent, and consent regarding adolescent HIV testing differs between states.23
Test kits and testing protocols vary between manufacturers. Visit the web version of this article for examples of FDA-approved (and CLIA-waived) test kits.24
TESTING PROCEDURES
As an example of rapid HIV screening, the procedure described here applies to the gathering and testing of specimens according to one manufacturer’s instructions.25 Consult the instructions and package inserts for the particular kit being used.
Test kits should be stored between 36° and 80° Fahrenheit. The tests should be performed in a room maintained at 59° to 99° F. If test kits or test kit controls are kept refrigerated, they must be left out long enough to return to operating temperature. The testing area must follow infection control standards, including the availability of a hazardous waste receptacle.
Prior to specimen collection, the patient must be fully informed and sign the proper documents. If collecting oral fluid, the patient may not have eaten, drunk, nor chewed gum for at least 15 minutes prior to the test, or used any oral care products within 15 minutes of testing. Otherwise, the dental team must wait 30 minutes prior to initiating the test. Once these conditions are met, the patient should open the pouch, remove the swab, and run it gently over the buccal surface of the gingiva, from upper right to upper left, and from lower left to lower right, keeping the swab away from the buccal mucosa and tongue. The swab is placed in the test vial and a timer is set for 20 minutes. The test results will be available in 20 minutes and should be read immediately. The result is valid for a period of 20 minutes to 40 minutes following completion of the test.
If collecting a blood sample by finger stick, the finger should be cleansed with an antiseptic wipe and the site allowed to dry. A sterile lancet is used to make the puncture. The first drop of blood should not be used; instead, the site should be wiped clean and the second drop captured with the loop. The blood-filled loop is inserted into the vial. After stirring, the loop is removed and discarded in a biohazard waste receptacle. The vial is now ready for the testing device found in the kit. As with the oral fluid testing method, results will be available in 20 minutes.
The indicator will show either reactive, nonreactive, or invalid. If invalid, the procedure should be repeated. If nonreactive, the patient should be informed and the test is complete. If reactive, the patient should be retested—and if saliva testing was performed, a blood test used. Patients who retest reactive should be referred for follow-up care. States may vary in their requirements for linkage to care, thus, it is best to consult state agencies for specifics regarding follow-up policies.
SUMMARY
Oral health professionals have the opportunity to be the first to identify the early stages of HIV infection. Rapid HIV testing can be completed in dental settings, with salivary assays generally requiring processing times of 30 minutes or less. Screening in the dental office identifies an individual as being preliminarily reactive or nonreactive. It is not a conclusive diagnostic test, and follow-up care must be arranged for patients who are preliminarily reactive. Providing dental patients with the option of a rapid HIV test is similar to screening for blood pressure or oral cancer. The opportunity to help improve patients’ lives is one that should not be overlooked.
REFERENCES
- Montagnier L. Historical essay. A history of HIV discovery. Science. 2002;298:1727–1728.
- U.S. Centers for Disease Control and Prevention.Estimated HIV incidence in the United States, 2007–2010. HIV SurveillanceSupplemental Report 2012;17(No. 4). Available at:cdc.gov/hiv/topics/surveillance/resources/reports/#supplemental. Accessed:September 13, 2017.
- U.S. Centers for Disease Control and Prevention.HIV in the United States: At a Glance. Available at:hiv.gov/hiv-basics/overview/data-and-trends/statistics. Accessed September 13,2017.
- U.S. Department of Health and Human Services. TheStages of HIV Infection. Available at:aidsinfo.nih.gov/understanding-hiv-aids/fact-sheets/19/46/the-stages-of-hiv-infection. Accessed September 13, 2017.
- Arts EJ, Hazuda DJ. HIV-1 antiretroviral drugtherapy. Cold Spring Harb Perspect Med. 2012;2:a007161.
- My VMC Virtual Medical Centre. AntiretroviralTherapy (Anti-HIV-Drugs). Available at:myvmc.com/treatments/antiretroviral-therapy-anti-hiv-drugs/#c3(April14,2017).Accessed September 13, 2017.
- World Health Organization. AntiretroviralTherapy. Available at: who.int/topics/antiretroviral_therapy/en/. AccessedSeptember 13, 2017.
- U.S. Centers for Disease Control and Prevention.HIV Risk Behaviors. Available at:cdc.gov/hiv/risk/estimates/riskbehaviors.html. Accessed September 13, 2017.
- Lab Tests online. HIV Viral Load. Available at:labtestsonline.org/understanding/analytes/
- viral-load/tab/test/%20%E2%80%9C. AccessedSeptember 13, 2017.
- NAM Aids Map. Viral load. Available at:aidsmap.com/Viral-load/page/ 1327496/. Accessed September 13, 2017.
- Bennett NJ. HIV Infection and AIDS. Available at:emedicine.medscape.com/ article/211316-overview. Accessed September 13, 2017.
- What is the National HIV/Aids Strategy? Availableat: hiv.gov/federal-response/national-hiv-aids-strategy/overview. AccessedSeptember 13, 2017.
- Leao JC, Ribeiro CM, Carvalho AA, Frezzini C,Porter S. Oral complications of HIV disease. Clinics (Sao Paulo).2009;64:459–470.
- Reznik DA. Perspective oral manifestations of HIVdisease. Top HIV Med. 2005;13:143–148.
- Vernillo AT, Caplan AL. Routine HIV testing indental practice: can we cross the rubicon? J Dent Educ.2007;71:1534–1539.
- San Francisco Aids Foundation. What is thedifference between HIV and AIDS. Available at:sfaf.org/hiv-info/basics/what-is-difference-between-hiv-aids.html. AccessedSeptember 13, 2017.
- Kumar P. Long term non-progressor (LTNP) HIVinfection. Indian J Med Res. 2013;138:291–293.
- U.S. Centers for Disease Control and Prevention.HIV Testing. Available at: cdc.gov/hiv/testing/index.html. Accessed September13, 2017.
- From DNA to RNA. In: Alberts B, Johnson A, LewisJ, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 4th ed.New York, New York: Garland Science; 2002.
- San Francisco AIDS Foundation. HIV Test WindowPeriods. Available at: sfaf.org/hiv-info/testing/hiv-test-window-periods.html.Accessed September 13, 2017.
- U.S. Centers for Disease Control and Prevention.CLIA Certificate of Waiver Fact Sheet. Available at:cdc.gov/hiv/testing/nonclinical/clia.html. Accessed September 13, 2017.
- U.S. Preventive Services Task Force. Final RecommendationStatement Human Immunodeficiency Virus (HIV) Infection: Screening. Availableat: uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/human-immunodeficiency-virus-hiv-infection-screening#consider.Accessed September 13, 2017.
- U.S. Centers for Disease Control and Prevention.Revised Recommendations for HIV Testing of Adults, Adolescents and PregnantWomen in Health-Care Settings. Available at: cdc.gov/mmwr/preview/mmwrhtml/rr5514a1.htm. Accessed Septemebr 13, 2017.
- U.S. Food and Drug Administration. Complete Listof Donor Screening Assays for Infectious Agents and HIV Diagnostic Assays.Available at:fda.gov/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/BloodDonorScreening/InfectiousDisease/ucm080466.htm#anti_HIV12_Assays.Accessed September 13, 2017.
- OraQuick ADVANCE Rapid HIV-1/2 Antibody Test Kitpackage insert. Available at: fda.gov/downloads/BiologicsBloodVaccines/ucm091917.pdf. Accessed September 13, 2017.
Featured image by ALEX LEVINE/ISTOCK/GETTY IMAGES PLUS
From Dimensions of Dental Hygiene. October 2017;15(10):49-52.



