Use of Fluoride Varnish in Caries Prevention
As this effective preventive tool is widely used, oral health professionals need to remain up-to-date regarding product innovations and the most current evidence-based approaches.
This course was published in the October 2017 issue and expires October 2020. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Identify the prevalence of caries in the United States.
- Explain the US Food and Drug Administration approval process and the American Dental Association recommendations concerning fluoride varnish.
- Discuss the clinical effectiveness of fluoride varnish in a comprehensive, customized fluoride program for individual patients.
- Compare and contrast the clinical effectiveness of fluoride varnish with other professional topical fluoride applications.
- Identify patient populations that will benefit from fluoride varnish application.
- Describe the various fluoride varnish formulations and delivery systems.
INTRODUCTION
The use of fluoride varnish—a highly concentrated form of topical fluoride—is on the rise. Fluoride varnish offers several benefits to manage patients’ oral health problems, and it is a simple and quick option to use in the dental office. Many fluoride varnishes are on the market today, with a variety of compositions and delivery systems. When confronted with the decision of which one to use in our clinics, one question remains: “Are all fluoride varnishes equal?” This article, “Use of Fluoride Varnish in Caries Prevention,” discusses the properties of fluoride varnishes to enlighten readers about their best options when it comes to treating patients. Also included is an overview of the regulatory aspect of fluoride varnishes, mechanism of action, and scientific evidence supporting the use of fluoride varnish. We hope this article delivers key information to facilitate your decision making when facing different clinical needs.
Fluoride varnish has many advantages over other professionally applied fluorides and remains a key component in the caries prevention armamentarium.
The use of fluoride varnish (FV) containing 5% sodium fluoride (NaF) has evolved significantly over the 23 years since it was first introduced in the United States, and, according to the American Dental Association (ADA) Council on Scientific Affairs, it is the primary choice for in-office caries prevention.1 While dental caries is highly preventable, it remains a prevalent, chronic disease in both children and adults.2 According to the US Centers for Disease Control and Prevention (CDC), from 2011 to 2014, 18.6% of children ages 5 to 19 had untreated caries, while 31.6% of adults ages 20 to 44 had untreated caries.3 The use of FV in a customized prevention program has become the most popular approach for the prevention of tooth decay and reduction of dentinal hypersensitivity. Additionally, the use of FV has expanded beyond the dental setting. In 47 states, primary care medical providers may apply FV to children with erupted teeth up to age 5 until a dental home is established.4 FV is also used in a variety of community health programs, such as Head Start and Special Supplemental Nutrition Programs for Women, Infants, and Children.4 The aim of this course is to provide information to assist oral health professionals in making evidence-based decisions regarding FV and other professional fluoride applications, as their roles and formulations evolve in a constantly changing marketplace.
Regulatory Report
In Europe, FV products have been available since the 1960s, but FV did not gain approval in the US until 1994.5 The US Food and Drug Administration (FDA) approved FV as a Class II Medical Device for use as a cavity liner and/or to treat dentinal hypersensitivity.4 Its use in the prevention of caries is considered “off label,” which means it is used in a manner not specified in the FDA’s approved packaging label. In this case, FV is used to address a disease (caries) that it is not approved to treat.4 FV’s off-label use is widely accepted in dentistry and has become preferred for caries prevention over other professional fluoride applications.1 Furthermore, both the ADA and the Canadian Dental Association include FV in their evidence-based clinical recommendations.1
A panel of experts convened by the ADA Council on Scientific Affairs presented its first evidence-based clinical guidelines regarding professionally-applied fluorides in 2006, which were updated and expanded in 2013 to include prescription-strength, home-use topical fluorides.1 The strength of the recommendations (based on the level of available evidence) ranges from “moderate” to “expert opinion,” and recommendations are categorized into three age groups (younger than 6; 6 to 18; and 18+). Only two professionally-applied agents are included in the recommendations: 2.26% FV ion (equates to 5% sodium fluoride or 22,600 ppm) and 1.23% acidulated phosphate fluoride (APF) gel, which equates to 12,300 ppm. The panel concluded that 2.26% FV ion is the only professional application recommended for children younger than 6 because FV has the lowest risk for ingestion and the fewest toxic effects of all professionally applied fluorides (gels, foams, rinses, or varnish).1 The expert panel also recommended the use of 2.26% FV ion or 1.23% APF gel for children ages 6 to 18, those older than 18, and for adults at risk of root caries. The recommendations include applications administered every 3 months to 6 months for individuals at elevated risk for caries. The recommendations state that patients at low risk of developing caries may not need additional topical fluorides, other than over-the-counter fluoride toothpaste and fluoridated water—the cornerstones of all fluoride programs. The ADA does not recommend the use of 0.1% FV or 1.23% APF foam.1 Table 1 outlines the current ADA recommendations for professional fluorides.
CHILDREN AND ADOLESCENTS
The body of evidence regarding FV suggests that it is safe and effective for patients of all ages with a variety of oral health challenges. A recently updated Cochrane Review of FV assessed the safety and efficacy of FV in preventing caries in child/adolescent patient populations.6 The review also set out to determine if the effect was influenced by the initial severity of caries and exposure to other sources of fluoride. The review concluded that FV applications between two times to four times per year resulted in a substantial caries-inhibiting effect in the primary and permanent dentition for children and adolescents, regardless of the caries risk level and exposure to other fluoride sources.6
Mishra et al7 conducted a systematic review of the role of FV in preventing early childhood caries (ECC). They reported a 5% to 63% reduction of ECC with twice per year application of FV and 48.3% to 55% reduction of ECC with three times per year application of FV.7 Another study by Autio-Gold and Courts8 concluded that FV can reverse active pit and fissure lesions in primary teeth.
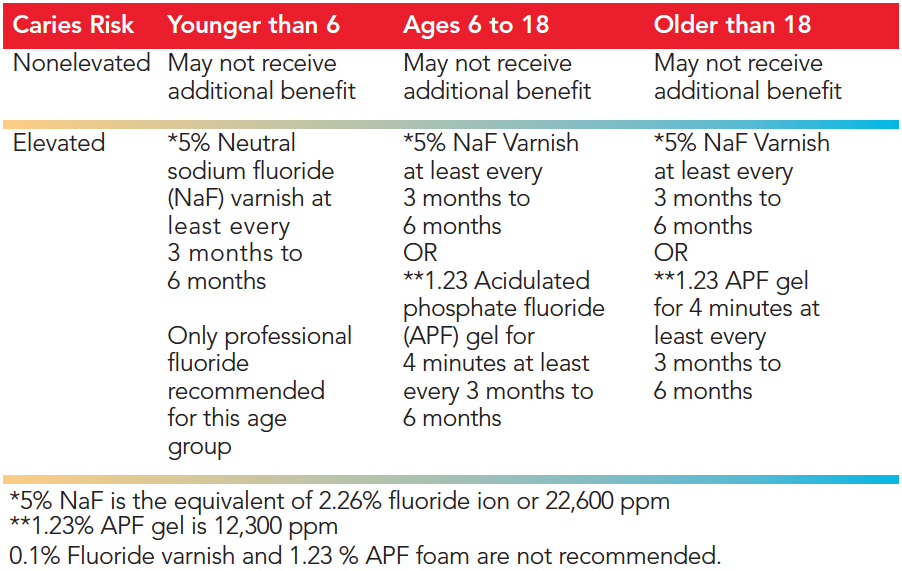 TABLE 1. American Dental Association Clinical Recommendations for Use Of Fluoride Varnish or Other Professionally Applied Fluorides
TABLE 1. American Dental Association Clinical Recommendations for Use Of Fluoride Varnish or Other Professionally Applied Fluorides
White Spot Lesions
A recent systematic review and meta-analysis conducted by Swiss researchers studied a variety of treatments for the reduction of orthodontically induced white spot lesions (WSL).9 They concluded that post-orthodontic monthly applications of FV, compared with other interventions, showed the most reduction in WSLs and the greatest improvement in esthetic appearance. It is theorized that the monthly FV protocol enhanced the natural remineralization of twice daily brushing with a fluoride dentifrice.9
Dentinal
For patients with exposed root surfaces, FV is a desensitizer and a viable intervention for preventing root caries. Ritter et al10examined the effect of one application of FV to sensitive tooth areas with a brush or a syringe. Both delivery methods were shown to reduce dentinal hypersensitivity for up to 24 weeks.10
A recent systematic review by Gluzman et al11 directly compared seven leading agents for the primary prevention and arrest (secondary prevention) of root caries: fluoride, chlorhexidine, xylitol, amorphous calcium phosphate (ACP), sealants, saliva stimulators, and silver diamine fluoride. The results provided strong evidence that the application of FV every 3 months was the best choice for the arrest of root caries.11 FV arrested 54% to 95% of early root lesions with an average rate of 78%.11 The literature supports the use of FV for a variety of patient needs. Table 2 lists patient populations that may benefit from FV application.
ADVANTAGES AND BENEFITS
Both 5% NaF varnish and 1.23% APF gel are recommended by the ADA for children older than 6; however, FV has many advantages over gel applications.1 FV is quick and easy to apply, adheres to moist surfaces, and does not require trays or saliva removal. It takes approximately 1 minute to wipe, dry, and then paint a thin layer of FV on a full dentition, whereas gels and foams must be applied in a tray for 4 minutes and require suction during and after removal.12 Additional advantages of FV include the fact it can be directly applied on areas like exposed root surfaces that traditional tray applications may not reach, and the sticky rosin (adhesive derived from pine trees) base helps it stay in contact with the tooth for much longer than gels or foams. Some FV brands migrate, allowing the fluoride to reach areas that cannot be reached with a brush, such as interproximally, and eliminates the need to paint every tooth surface. FV can be removed after 4 hours to 6 hours and up to 24 hours, depending on brand, after application by toothbrushing. In one study, the authors concluded that FV was more accepted than fluoride foam because varnish required “less time and less discomfort” during application in children ages 3 to 6, especially in patients with a severe gag reflex.13 FV is unlikely to contribute to toxicity or dental fluorosis, because less overall amounts are used, and any potential ingestion is spread over 24 hours.12
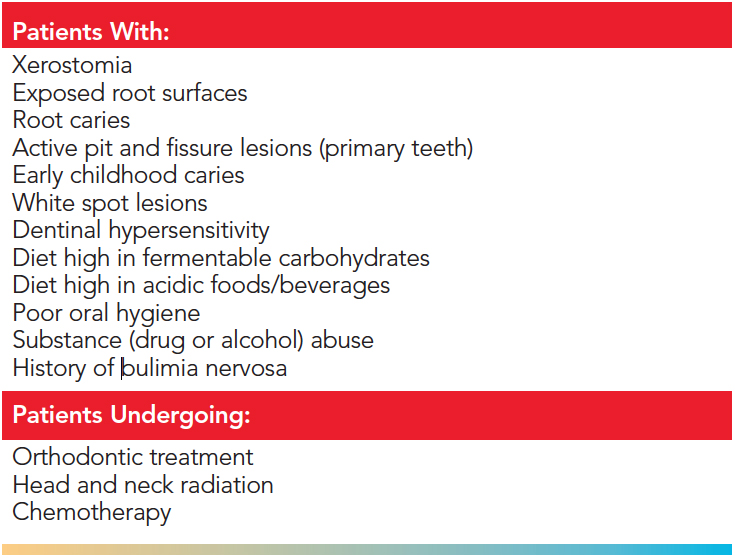 TABLE 2. Patients Who May Benefit from a 5% Sodium Fluoride Varnish Application
TABLE 2. Patients Who May Benefit from a 5% Sodium Fluoride Varnish Application
Fluoride gel application amounts include 2.0 ml per tray for the primary dentition and no more than 2.5 ml per tray for the permanent dentition.12 More fluoride is likely to be swallowed with gel because it requires a larger amount of the product to be applied compared with FV. Patients are required to wait at least 30 minutes before eating or drinking after a gel or foam fluoride treatment.
Manufacturer instructions should be reviewed, but most FV post-operative instructions state the patient can eat or drink immediately, which is another advantage FV has over gels and foams. Patients should avoid any hard, sticky, hot, or crunchy foods and any hot or alcoholic beverages for 4 hours to 6 hours after application.
Research shows that the longer the fluoride is in contact with the tooth, the better the outcomes.14 However, to best serve noncompliant patients, a FV has been introduced that offers a fast release of fluoride. It is formulated to release most of its fluoride within the first 2 hours of application through special components in the film-forming agent and the solvents used.15 This helps patients who are prone to brushing FV off before its full application time has been reached to still reap the benefits of the fluoride contact time.
Mechanism of Action
FV was developed to prolong contact between fluoride and the tooth. It works to reduce dentinal hypersensitivity and prevent caries. FV treats cervical dentin hypersensitivity by precipitating calcium fluoride on the tooth surface to form a barrier to occlude open dentinal tubules. The occlusion of the tubules reduces fluid flow through them, ultimately, reducing hypersensitivity.10 The natural rosin in FV may provide an additional barrier.
In the prevention of caries, fluorides work topically to promote remineralization of the tooth after an acid attack, inhibit demineralization by lowering the critical pH at which the tooth begins to demineralize, and reduce the production of acid by cariogenic bacteria. The first two mechanisms require the presence of calcium and phosphate to combine with the fluoride. FV as a caries-preventive intervention is proposed to form globules of calcium and fluoride ion pairs (CaF+) on the tooth to create a reservoir. These CaF+ reservoirs are retained in plaque and slowly released to enhance remineralization and inhibit demineralization of the tooth. The formation of CaF+ reservoirs is dependent on the availability of calcium found as an additive in the FV or in saliva and the oral environment. Some patients do not have adequate amounts of saliva to provide enough calcium and phosphate to effectively combine with the fluoride available in the FV. Therefore, some manufacturers are adding calcium and phosphate to FV formulations to make calcium ions bioavailable and to, hopefully, increase efficacy.16 When calcium ions are added to enhance FV formulation, the premature reaction between fluoride and calcium ions must be avoided. This premature reaction during storage might render the minerals less available at the tooth surface, and therefore less effective in preventing caries. 16
Formulations
 FIGURE 1. Fluoride varnish products come in a variety of delivery systems, typically including a brush for application.
FIGURE 1. Fluoride varnish products come in a variety of delivery systems, typically including a brush for application.
Many FVs are adding minerals to their formulations, most commonly calcium and phosphate, to combine with fluoride to create fluoroapatite, a strong acid-resistant mineral. (Table 3). These products are designed to enhance the anti-cariogenic efficacy of varnishes compared with those that don’t contain minerals. Calcium and phosphate must both be bioavailable in the FV product to increase fluoride release and the remineralization process.16 Several agents are used, including: tri-calcium phosphate modified by fumaric acid; a complex of both casein phosphopeptide and ACP (Recaldent); calcium and phosphate with xylitol; calcium sodium phosphosilicate (Novamin); sodium trimetaphosphate; and nano-hydroxyapatite (nonorganic crystals composed of both calcium phosphate and calcium carbonate).
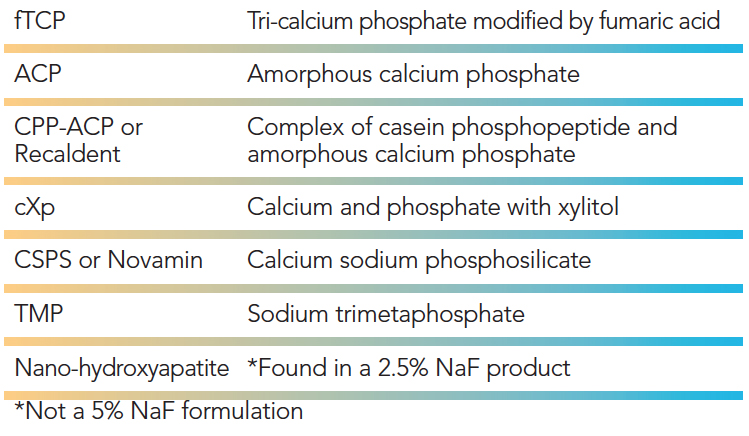 TABLE 3. Mineral Additives Found in 5% Sodium Fluoride (NaF) Varnish Formulations.
TABLE 3. Mineral Additives Found in 5% Sodium Fluoride (NaF) Varnish Formulations.
Varying types of research have been conducted to determine the efficacy of novel FV formulations with enhancements. As is the case with many other oral care and medical products, the studies on these formulations largely use an in vitro or laboratory protocol, which may not best represent the intraoral environment such as interactions with the salivary pellicle, proteins, buffers, and micro-organisms, as well as oral hygiene.17 In vivo studies are expensive, time consuming, and require human subjects. Results of these in vitro studies vary widely and researchers recommend that additional in vivo studies are needed to review the efficacy of newer FV formulations to help providers make evidence-based treatment choices for their patients and to establish FV efficacy guidelines.18–20It is important to note there is no minimum standard or ADA recommendation for FV formulations with mineral enhancements.
FV is now available in varying colors (yellow, white, and clear) and numerous flavors. Some FV comes in a blister pack, palette, or well, along with a brush for application (Figure 1). Other varnishes come in small unit dose tear-off packages. Oral health professionals need to read the manufacturer directions and be familiar with the FV product prior to use.
Future Perspectives
Five percent NaF varnish continues to play a significant role in an overall fluoride program that includes the daily consumption of fluoridated water and twice daily brushing with fluoride toothpaste. FV provides many advantages over other professionally applied fluorides. Clinicians should use scientific research, experience, patient preferences, and clinical circumstances to guide their decision making. From contact time to migration properties to delivery system, there are many choices in FV products. Oral health professionals are prudent to evaluate the features of different FV products to find the one that best fits their clinical needs.
REFERENCES
- Weyant, RJ, Tracy SL, Anselmo TT, Beltran-Aguilar, ED. American Dental Association Council on Scientific Affairs Expert Panel on Topical Fluoride Caries Preventive Agents. Topical fluoride for caries prevention: executive summary of the updated clinical recommendations and supporting systematic review. J Am Dent Assoc. 2013;144:1279–1291.
- National Institute of Dental and Craniofacial Research. Tooth Decay (Caries). Available at: nidcr.nih.gov/OralHealth/Topics/ ToothDecay/. Accessed September 14, 2017.
- United States Centers for Disease Control and Prevention. Oral and Dental Health. Available at: cdc.gov/nchs/fastats/ dental.htm. Accessed September 14, 2017.
- Association of State and Territorial Dental Directors Fluorides Committee. Fluoride Varnish: an Evidence-Based Approach Research Brief. Available at: astdd.org/docs/fl-varnish-brief-september-2014-amended-05-2016.docx. Accessed September 14, 2017.
- Marya C, Dahiya V. Fluoride varnish: a useful dental public health tool. Internet Journal of Dental Science. Available at: ispub.com/IJDS/4/2/6304. Accessed September 14, 2017.
- Marinho VC, Worthington HV, Walsh T, Clarkson JE. Fluoride varnishes for preventing dental caries in children and adolescents. Cochrane Database Syst Review. 2013;7:CD002279.
- Mishra P, Fareed N, Battur H, Khanagar S, Bhat MA, Palaniswamy J. Role of fluoride varnish in preventing early childhood caries: A systematic review. Dent Res J (Isfahan). 2017;14:169–176.
- Autio-Gold JT, Courts F. Assessing the effect of fluoride varnish on early enamel carious lesions in the primary dentition. J Am Dent Assoc. 2001;132:1247–1253.
- Höchli D, Hersberger-Zurfluh M, Papageorgiou SN, Eliades T. Interventions for orthodontically induced white spot lesions: a systematic review and meta-analysis. Eur J Orthod. 2017;39:122–133.
- Ritter AV, Dias WL, Miguez P, Caplan D, Swift EJ. Treating cervical dentin hypersensitivity with fluoride varnish. J Am Dent Assoc. 2006;137:1013–1020.
- Gluzman R, Katz RV, Frey BJ, McGowan R. Prevention of root caries: a literature review of primary and secondary preventive agents. Spec Care Dentist. 2013;33:133–140.
- Wilkins EM. Clinical Practice of the Dental Hygienist. 12th ed. Philadelphia: Wolters Kluwer; 2017:607–608.
- Hawkins R, Noble J, Locker D, et al. A comparison of the costs and patient acceptability of professionally applied topical fluoride foam and varnish. J Public Health Dent. 2004;64:106–110.
- Carey CM. Focus on fluorides: update on the use of fluoride for the prevention of dental caries. J Evid Based Dent Pract. 2014;(14 Suppl):95–102.
- Zehrer C, Klaiber PR, Russell VA, et al. Patient perceptions of three fluoride varnish products. J Dent Res. 2017;96(Special Issue A):2023.
- Shen P, Bagheri R, Walker GD, et al. Effect of calcium phosphate addition to fluoride containing dental varnishes on enamel demineralization. Aust Dent J. 2016;61:357–365.
- Manarelli MM, Delbem ACB, Baez-Quintero LC, N. de Moraes FR, Cunha RF, Pessan JP. Fluoride varnishes containing sodium trimetaphosphate reduce enamel demineralization in vitro. Acta Odontol Scand. 2017;75:376–378.
- Majithia U, Venkataraghavan K, Choudary P, Trivedi K, Shah S, Virda M. Comparative evaluation of application of different fluoride varnishes on artificial early enamel lesion: An in vitro study. Indian J Dent Res. 2016;27:521–527.
- Al Dehailan L, Martinez-Mier EA, Lippert F. The effect of fluoride varnishes on caries lesions: an in vitro investigation. Clin Oral Invest. 2016;20:1655–1662.
- Al Dehailan L, Lippert F, Gonzalez-Cabezas C, Eckert GJ, Martinez-Mier EA. Fluoride concentration in saliva and biofilm fluid following the application of three fluoride varnishes. J Dent. 2017;60:87–93.
Featured image by NFSPHOTO/ISTOCK/GETTY IMAGES PLUS
From Dimensions of Dental Hygiene. October 2017;15(10):53-58.



