 JARUN011/ISTOCK/THINKSTOCK
JARUN011/ISTOCK/THINKSTOCK
Hepatitis C—A Silent Killer
With increasing numbers of hepatitis C virus infections, oral health professionals need to advise their baby boomer patients to seek testing.
Hepatitis is a viral infection caused by one of several common strains of the hepatitis virus A through E. A serious threat to health, hepatitis infection can lead to inflammation of the liver, with a possible progression to cirrhosis, liver failure, and cancer.1 This article will focus on the hepatitis C virus (HCV), which was known as non-A non-B hepatitis until 1992 when it was classified as HCV, after which serological testing was developed.2
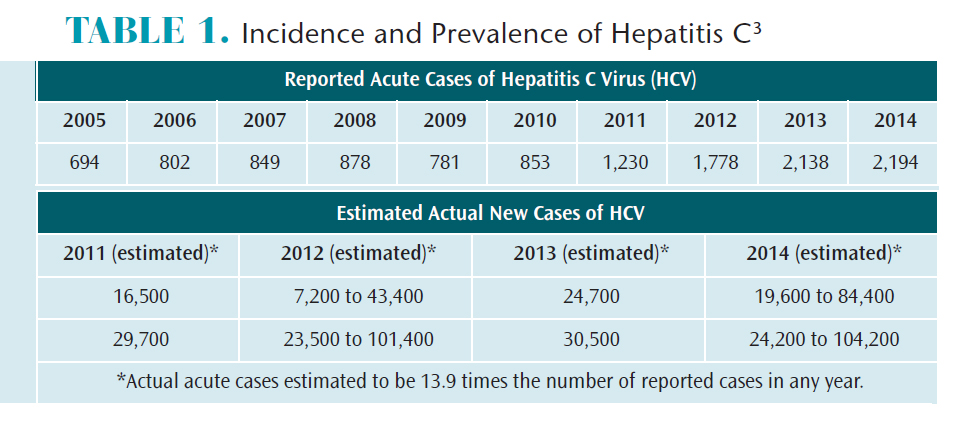
Prior to 1965, the incidence of HCV was very low, with only 18 cases per 100,000 individuals. By the 1980s, this number had increased to 130 cases per 100,000.2 Between 1992 and 2004, new cases of HCV declined and remained steady for many years. Some of this decline was attributed to safer injection practices among intravenous (IV) drug users.2 Beginning in 2011, however, the number of HCV cases has risen each year (Table 1).3
In 2014, 19,659 deaths were attributed to HCV.4 Mortality related to HCV continues to increase. In 2007, HCV caused 15,106 deaths—more than the 12,734 deaths attributed to human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS).5Currently, individuals age 55 to 64 account for more than half of HCV deaths in the US.2
TRANSMISSION
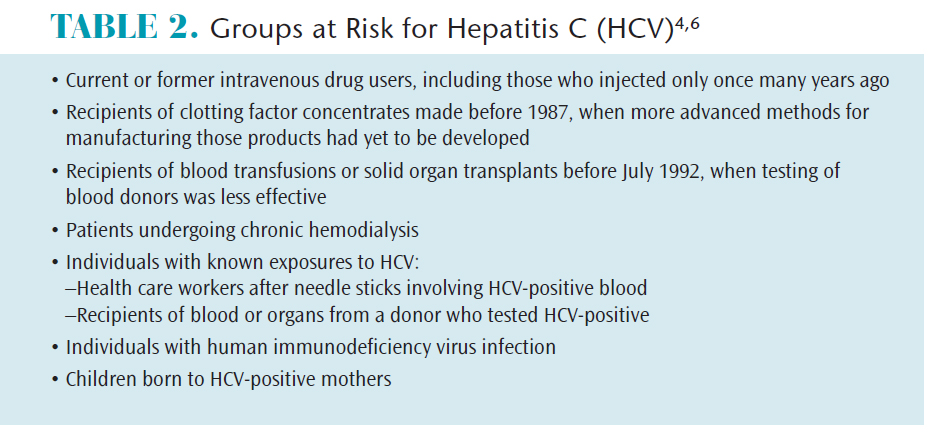
HCV transmission occurs through exposure to blood contaminated with the virus, generally through multiple percutaneous exposures.6 The sharing of needles and syringes among current and former IV drug users is the most common culprit.4 Transmission may also occur via sexual contact; childbirth; sharing of personal items, such as razors and toothbrushes; and occupational exposures, such as sharps injuries.4,6 Groups at highest risk for contracting HCV include: former and current IV drug users; those on long-term dialysis; recipients of blood transfusions and organ transplants prior to 1992; health care workers; infants born to infected mothers; and those with HIV (Table 2).4,6
Early detection is challenging due to the asymptomatic nature of HCV, with some cases remaining undiagnosed for as many as 20 years or more. Asymptomatic patients are at high risk for cirrhosis and liver cancer because they might not be diagnosed for several years or even decades, by which time irreversible liver damage may have already occurred.6 Those at risk for HCV infection must be screened and tested early. The CDC recommends HCV testing for those at increased risk, such as baby boomers born between 1945 and 1965, current and former IV drug users, recipients of blood transfusions or organ transplants prior to 1992, individuals with HIV, patients on long-term dialysis, patients with liver disease, and children born to HCV-infected mothers.6 Testing is also recommended for those with known exposure—particularly health care workers after occupational exposure incidents, such as sharps injuries involving HCV-infected blood.6
HEPATITIS C VIRUS TESTING
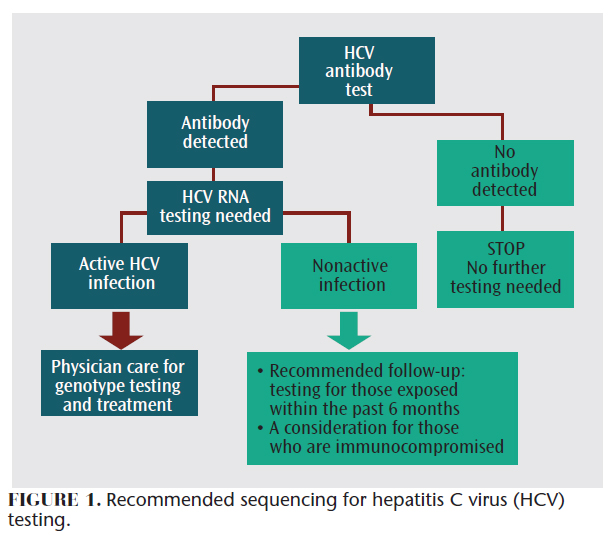
Individuals at risk for HCV infection should follow the recommended sequencing for testing (Figure 1). Initially, laboratory testing was required to detect HCV infection. In 2010, the US Food and Drug Administration (FDA) approved a rapid HCV antibody test, which has similar sensitivity and specificity as the FDA-approved anti-HCV laboratory testing methods.7 The rapid antibody testing can be performed with finger capillary blood samples or venipuncture samples. The ease of use associated with this method enables HCV testing to occur in nontraditional settings, such as medical offices and public health departments. There are limitations, however, to both rapid and laboratory-based testing methods. These include the inability to determine if the positive results for antibodies are related to a current HCV infection or a past exposure. HCV RNA testing is required for a positive anti-HCV result to determine active or nonactive HCV infection.
CENTERS FOR DISEASE CONTROL AND PREVENTION RECOMMENDATIONS
Lengthening the life spans of those infected with HCV depends on early diagnosis and treatment.2 The 1998 CDC recommendations for HCV testing included all individuals with an increased risk of exposure to HCV. Due to the increased HCV incidence and mortality in the baby boomer generation, the CDC updated the recommended HCV testing guidelines in 2012 to include all individuals born during 1945 and 1965, regardless of known risk. Following a review of the literature, the US Preventive Service Task Force also recommended a one-time HCV screening for those born between 1945 and 1965.8 Increased risk factors for this generation are related to the lack of widespread testing of blood and organs prior to 1992.2 Oral health professionals should follow the CDC recommendations and highly encourage patients born between 1945 and 1965 to follow the recommended testing sequence for identification of asymptomatic HCV infections.
TREATMENT OF HEPATITIS C INFECTION
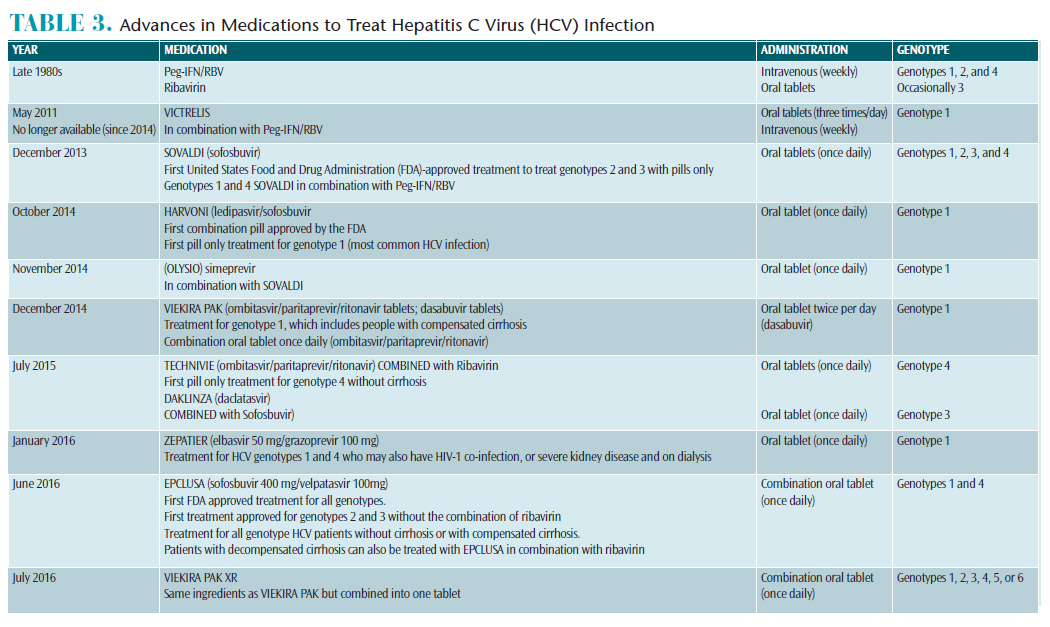
Once the diagnosis of active HCV is determined, patients can start the recommended antiviral treatments (Table 3). The first step prior to beginning treatment is to receive hepatitis A and hepatitis B vaccinations and screening for alcohol consumption/abuse. Then, the physician will recommend a genotype-specific HCV treatment. Over the past several years, there have been substantial advances in HCV antiviral treatments. Originally, intravenous pegylated interferon (alone or combined with ribavirin) was the only choice for HCV treatment. Interferon had significant side effects such as anemia, fever, headache, muscle pain, anxiety/depression, and gastrointestinal symptoms, causing many people to discontinue treatment.9 Since 2011, various FDA-approved medications—specific to genotype—have provided shorter treatment durations, reduced side effects, and increased cure rates for individuals with active HCV.9 Currently, epclusa is an HCV antiviral medication for genotypes 1 to 6 that is taken once daily for 12 weeks (can be administered at a 24-week dosage). This new medication demonstrated a 98% cure rate in individuals with genotypes 1 to 6 without cirrhosis and an 83% cure rate in individuals with decompensated cirrhosis. Rates for individuals with decompensated cirrhosis increased to 94% when epclusa was combined with ribavirin.9 This new antiviral medication can seriously slow the heart rate. Death can occur if all health conditions and current medications related to decreased heart rate are not identified prior to treatment.9Advances in treatment strategies are encouraging for individuals with HCV infection. Oral health professionals should inform patients born between 1945 and 1965 of the new successful HCV treatment options and identify the benefits of early testing.
IMPLICATIONS FOR ORAL HEALTH PROFESSIONALS
To reduce the risk of bloodborne disease transmission, such as HCV, oral health professionals must use standard precautions for safety in the workplace. The 2003 CDC Guidelines for Infection Control in Dental Health-Care Settings provide comprehensive guidance regarding standard precautions that include proper hand hygiene, personal protective equipment, safe work behaviors and safety devices, vaccination for hepatitis A and hepatitis B, and treating all patients as infectious.10 There is no vaccine for HCV at this time, so the use of standard precautions is required.
CONCLUSION
HCV is a silent killer, with most liver injury occurring without symptoms until the damage is significant. Identification is now easily accessible to patients with a rapid test and advancements in oral antiviral medications offer higher cure rates with shorter treatment durations.9 Oral health professionals must be knowledgeable about HCV in order to safely treat patients.
References
- Centers for Disease Control and Prevention. Viral Hepatitis-Hepatitis C Information. Available at: cdc.gov/hepatitis/hcv/index.htm. Accessed December 16, 2016.
- Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61(RR–4):1–32.
- Centers for Disease Control and Prevention. Statistics and Surveillance. Available at: cdc.gov/hepatitis/hcv/statisticshcv.htm. Accessed December 16, 2016.
- Centers for Disease Control and Prevention. The ABCs of Hepatitis. Available at: cdc.gov/hepatitis. Accessed December 16, 2016.
- Centers for Disease Control and Prevention. Surveillance for Viral Hepatitis-United States 2014. Available at: cdc.gov/hepatitis/statistics/2014surveillance/ commentary.htm#summary. Accessed December 16, 2016.
- Centers for Disease Control and Prevention. Hepatitis C: FAQs for Health Professionals. Available at: cdc.gov/hepatitis/hcv/hcvfaq.htm. Accessed December 16, 2016.
- Centers for Disease Control and Prevention. Testing for HCV infection: An update of guidance for clinicians and laboratorians. MMWR Morb Mortal Wkly Rep. 2013;62:362–365.
- United States Preventive Service Task Force. Final Update Summary: Hepatitis C Screening. Available at: uspreventiveservicestaskforce.org/Page/Document/ UpdateSummaryFinal/hepatitis-c-screening. Accessed December 16, 2016.
- American Liver Foundation. Advances in Medications to Treat Hepatitis C. Available at: hepc.liverfoundation.org/treatment/the-basics-about-hepatitis-c-treatment/advances-in-medications. Accessed December 16, 2016.
- Kohn WG, Collins AS, Cleveland JL, et al. Guidelines for infection control in dental health-care settings—2003. MMWR Recomm Rep. 2003;52(RR–17):1–61.
From Dimensions of Dental Hygiene. January 2017;15(1): 29-30, 32-33.

