 ROBERTODAVID / ISTOCK / GETTY IMAGES PLUS
ROBERTODAVID / ISTOCK / GETTY IMAGES PLUS
Evaluating the Risks Posed By Ultrasonic Aerosols
Multilevel infection control steps are needed to provide safe and effective dental care.
The global pandemic has increased interest in the dangers of “aerosol-generating procedures.” This term originally referred to aerosol procedures directly associated with the hospital care of patients with acute COVID-19. Specifically, study was first focused on the risks of aerosols when removing breathing tubes and clearing the airways of patients with COVID-19 on forced air respirators because when the airway tube is removed, the patient invariably coughs and aerosolizes large volumes of material from the trachea and pharynx. Due to the rapid spread of COVID-19 to healthcare workers and the confirmed presence of SARS-CoV-2 in this airborne material, the decision was made to label all aerosol-generating procedures as dangerous.
Given this background, all spray produced during dental procedures was also thought of as potentially dangerous. Initially, any procedure that produced aerosols—such as wet drilling and ultrasonic scaling—was banned. Later, severe restrictions on dental aerosol-generating procedures were implemented. As knowledge of COVID-19’s epidemiology has grown and SARS–CoV-2 mutates to strains that cause less severe disease, the question arises as to how dangerous dental aerosol-generating procedures actually are to healthcare workers and what infection control procedures are indicated as we transition into the so-called “COVID normal” world. This article will discuss the current understanding of dental aerosol-generating procedures in general, but will concentrate on the aerosols produced during the use of ultrasonic scalers.
Contamination of Ultrasonic Aerosols
Aerosols produced during ultrasonic scaling procedures are always contaminated and are, therefore, potentially dangerous. There are two sources of contamination inherent in dental ultrasonic aerosols:1
- The instrument itself, including its source of irrigation/cooling fluid
- Material found in the patient’s mouth
Ultrasonic scalers as a source of contamination are negligible. The handpiece and the scaler tip that comes in contact with the patient are routinely sterilized between patients. For many modern ultrasonic units, the tubing and connection between the handpiece and the console of the instrument can also be sterilized. When both portions of the ultrasonic instrument are sterile, the instrument itself as a source of infection is eliminated. In some older units, the handpiece and tubing can’t be sterilized. These units should be replaced with sterilizable units as soon as possible.
The greater instrument-related infection control concern is the water source for irrigation and cooling of the handpiece and tip. The ideal source of irrigation is sterile water, such as a disposable intravenous solution bag or a nondisposable sterile solution source, that feeds into the ultrasonics’ water system and can be autoclaved between patients. Unfortunately, outside of a few very expensive units primarily designed for ultrasonic surgical cutting, ultrasonic scalers that follow this protocol are difficult to find.
Most ultrasonic scalers either have an independent nonsterile water source or are directly connected to municipal water sources. Both of these irrigation sources can deliver very clean but not sterile water to the ultrasonic scaler. If these water sources are not scrupulously maintained, they can become grossly contaminated, creating a major source of contamination for oral health professionals as well as patients. Nonsterilizable water sources should be cleaned and serviced daily following the manufacturer’s instructions with a more in-depth cleaning performed at least weekly. Unfortunately, these maintenance cleaning steps are frequently not followed, leading to gross contamination of the ultrasonic spray.
Contamination From the Patient
The greatest source of contamination of the spray and aerosols produced by ultrasonic scalers is from the operating site. In other words, if the instrumentation and irrigation water source are sterilized and maintained, the spray and aerosol from an ultrasonic scaler is very safe. That all changes when the ultrasonic tip is placed in the patient’s mouth and used to remove calculus and biofilm.
Some contamination from saliva can be reduced using a preprocedural mouthrinse. However, calculus and biofilm, by their very nature, are filled with bacteria and virus particles (bioburden) that live in the patient’s mouth. 2 The protein-binding material in the bioburden is protective against the disinfecting effect of preprocedural mouthrinses so that infectious material remains after rinsing. When calculus and bioburden are removed by the ultrasonic tip, a portion of the bioburden containing bacteria and viruses is mixed with the irrigation solution and becomes airborne. This airborne bioburden—whether the patient has COVID-19 or not—is always a risk for the oral health professional and potentially for other patients in the office.
Are Ultrasonic Aerosols Dangerous?
The aerosols generated by an ultrasonic scaler always represent a danger. This was recognized by the United States Centers for Disease Control and Prevention nearly 20 years ago.3 The use of high-volume evacuation (HVE) as an engineering control within the operative site and “adequate” protective equipment (PPE) is required when using an ultrasonic scaler. Since the advent of COVID-19, this is a requirement and not a suggestion.4 The use of HVE is now necessary to meet federal Occupational Safety and Health Administration (OSHA) regulations.5 These guidelines are relatively easy to follow, and, for many, compliance has become routine during ultrasonic usage. The question for most oral health professionals is what equipment is needed. The remainder of this article will discuss the various approaches to compliance as they apply to ultrasonic scalers.
Environmental Devices
Many products are designed to reduce contamination of the atmosphere in the operatory and the entire dental office. These include high-efficiency particulate absorbing (HEPA) filters, various ultraviolet (UV) light systems, external evacuators, and positive flow ventilation. These devices are useful but do not address the direct reduction of contamination at its source—the treatment site in the patient’s mouth. They instead address any contamination that has escaped from the immediate operating site. These tools, in various forms and in specific circumstances, have been part of hospital infection control for many years. The least expensive is the HEPA filter, but it requires frequent maintenance and filter element replacement to be moderately effective. The most effective and most expensive are “upstairs” UV light units and positive flow ventilation. None of these devices are currently part of OSHA’s requirements for dental offices but their use may be mandated in the future.

Personal Protective Equipment
A large percentage of dental personnel comply with the existing requirements for PPE. Most consider the minimal level of PPE to be gloves, head covering, eye protection, “adequate” masking, and disposable single patient-use gowns. Initially, N95 filtration level masks were considered mandatory but recent research has shown that standard surgical masks may be adequate for protection.6 The level of mask filtration necessary remains a question, with the safest approach being the continued use of N95 masks.
High-Volume Evacuation During Ultrasonic Scaling
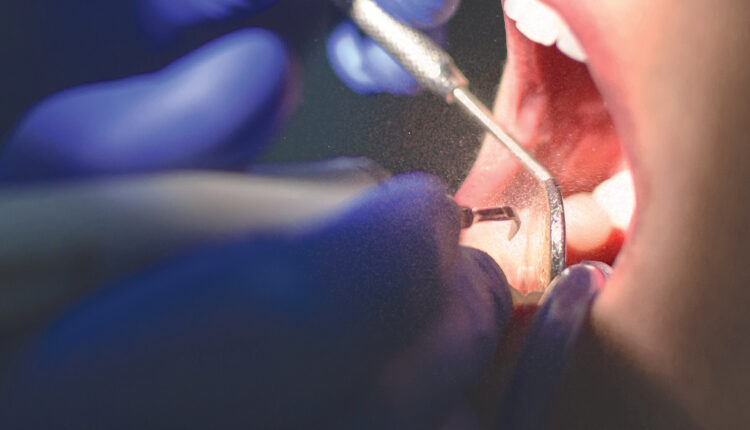
The greatest failure in the control of contaminated aerosols is the fact that many dental hygienists use ultrasonic scalers without adequate HVE. Again, sufficient HVE must be used during ultrasonic scaling. A saliva ejector is not an HVE, and the use of only a saliva ejector is inadequate for controlling contaminated ultrasonic aerosols and does not comply with infection control standards (Figure 1).1
The ideal solution is to have a trained dental assistant use a large bore HVE suction tip during ultrasonic scaling. A well-trained dental assistant is expert at keeping the tip of the HVE within 2 cm of the scaling tip. This is the ideal location for removing contaminated aerosols within the patient’s mouth. The goal is to prevent the contamination from escaping into the atmosphere of the operatory or directly into the operator’s face. Once the contaminated aerosols escape the enclosed environment of the patient’s mouth, they become a greater danger for all oral health professionals as well as other patients.
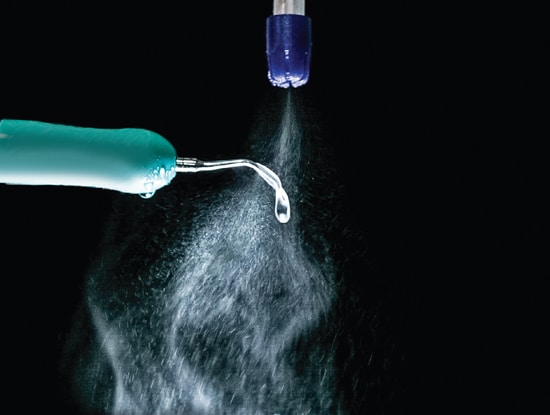
Unfortunately, due to economic considerations, most dental hygienists continue to work without an assistant. In this situation, several acceptable HVE options for controlling contaminated aerosols are available. HVE equipment that attaches to the ultrasonic scaler handle (Figure 2) or inserts in the patient’s mouth is the only option that fulfills the need to control contaminated aerosols before they escape the patient’s mouth. Only the HVE that attaches to the ultrasonic handle has extensive research documenting its effectiveness.7
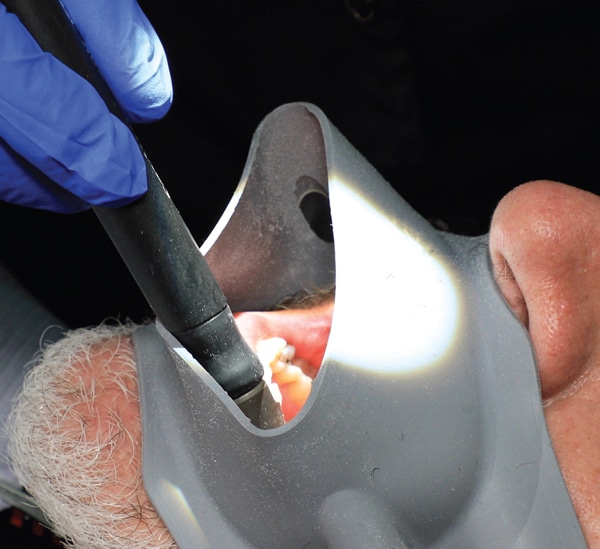
Some devices are designed to catch the contaminated aerosols just after they escape the patient’s mouth but prior to the contamination reaching the operator or the operatory environment. These generally have a large-bore HVE positioned on a device that the patient bites on or fits around his or her mouth. These options are often heavy and may need to be frequently repositioned in order to maintain the close approximation to the scaler tip necessary for adequate aerosol control. A lightweight device with flexible edges that surrounds the entire circumference of the mouth and allows easier operator access is shown in Figure 3. There is little research on any of these external devices verifying their ability to reduce contamination. It is always preferable to control the contamination within the mouth rather than after it enters the environment.
COVID-19 Pandemic
The greatest recent influence on the delivery of dental care has been the COVID-19 pandemic. Fortunately, the level of COVID-19 cases among dental professionals is low and similar to that of the general population.8,9 The epidemiology of COVID-19 seems to be following a standard curve for infectious diseases. This curve starts with a highly infectious agent—currently SARS-Cov-2—which causes severe symptoms and leads to death in susceptible and immune-compromised individuals. This was the case in early 2020 when all nonemergency dental procedures were prohibited. As the disease progressed over time, two well-known epidemiologic phenomena occurred:
- The most susceptible patients got the disease and either developed natural immunity or succumbed to it
- The virus mutated to a less damaging (less fatal) form
This has led to our current situation, which some call “COVID normal.” This means that COVID-19, like influenza, will remain in circulation. Most individuals will either have natural or vaccinated immunity, which will diminish symptom severity and disease fatality, but will not stop transmission of the disease.
Conclusion
While “COVID normal” will alleviate much of the inherent fear felt by healthcare workers early in the disease cycle, the danger of becoming infected remains, and even a mild case of COVID-19—like the flu—is to be avoided. This means that the need for the aforementioned multilevel infection control steps need to be routine for all dental care. These steps are extremely important during the use of an ultrasonic scaler due to the fact that it produces copious amounts of contaminated aerosols—posing a risk to oral health professionals.
The use of an HVE during ultrasonic scaling that is effective for “in-the-mouth” contaminated aerosol reduction and strict compliance with PPE requirements are mandatory. Inadequate measures, such as depending on a saliva ejector, must be avoided. Devices that enable oral health professionals to protect themselves and their patients from contaminated aerosols, allowing them to deliver excellent dental care, should be implemented.
References
- Harrel SK, Molinari J. Aerosols and splatter in dentistry: a brief review of the literature and infection control implications. J Am Dent Assoc. 2004;135:429–437.
- Natto ZS, Afeef M, Bakhrebah MA, et al. Can periodontal pockets and caries lesions act as reservoirs for coronavirus? Mol Oral Microbiol. 2022;37:77–80.
- Kohn WG, Collins AS, Cleveland JL, et al. Guidelines for infection control in dental health-care settings—2003. MMWR Recomm Rep. 2003;52(RR-17):1–61.
- United States Centers for Disease Control and Prevention. Interim Infection Prevention and Control Recommendations for Healthcare Personnel During the Coronvirus Disease 2019 (Covid-19) Pandemic. Available at: cdc.gov/coronavirus/떓-ncov/hcp/infection-control-recommendations.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fhcp%2Fdental-settings.html. Accessed May 22, 2022.
- United States Department of Labor. COVID-19 Control and Prevention, Dentistry Worker and Employees. Available at: osha.gov/coronavirus/control-prevention/dentistry. Accessed May 22, 2022.
- Infectious Diseases Society of America. Infectious Diseases Society of America Guidelines on Infection Prevention in Patients With Suspected or Known COVID-19. Available at: idsociety.org/practice-guideline/covid-19-guideline-infection-prevention. Accessed May 22, 2022.
- Harrel SK, Barnes JB, Rivera-Hidalgo F. Reduction of aerosols produced by ultrasonic scalers. J Periodontol. 1996;67:28–32.
- Estrich CG, Gurenlian JR, Battrell A, et al. Infection prevention and control practices of dental hygienists in the United States during the COVID-19 Pandemic: a longitudinal study. J Dent Hyg. 2022;96:17–26.
- Madathil S, Siqueira WL, Marin LM, et al. The incidence of COVID-19 among dentists practicing in the community in Canada: A prospective cohort study over a 6-month period. J Am Dent Assoc. 2022;153:450–459.
From Dimensions of Dental Hygiene. June 2022; 20(6)10-13.

