MUTLU KURTBAS/E+/GETTY IMAGES PLUS
MUTLU KURTBAS/E+/GETTY IMAGES PLUS
Emergency Medicine Preparedness
The entire dental team needs to be equipped to identify and manage perioperative emergencies during moderate sedation.
This course was published in the January 2022 issue and expires January 2025. The author has no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Explain patient risk and various perioperative emergencies that may arise from use of moderate sedation in dental therapy.
- List steps dental teams can take to prepare for, and avoid, medical emergencies during moderate sedation.
- Discuss specific responses that may be warranted in an emergent situation arising from moderate sedation during dental care.
Advances in medicine have enabled patients with incapacitating conditions to become medically stable enough to receive dental treatment. However, these patients may be at greater risk for perioperative emergencies due to underlying comorbidities. Because of this, moderate sedation can be useful for the prevention of many emergent events. Moderate sedation is an inherently safe treatment modality largely because consciousness is continuously maintained, anxiety is reduced, and the stress response is mitigated. Clinicians need to identify potentially reactive patients, anticipate problems, and be prepared to manage perioperative emergencies. While this paper reviews the most common adverse events that may be encountered, further reading is recommended.1–3
Most anesthetic-related morbidity and mortality occur due to improper airway management, but this can be minimized by maintaining patient consciousness. This means every patient, regardless of medical status, is at risk when deeper levels of sedation are inadvertently encountered. Comprehensive preoperative patient assessment and medical consultation (if required) are crucial for prevention of anesthetic-related emergencies. The majority of life-threatening emergencies can be prevented by thoroughly evaluating the stability of a patient’s general condition.
Preparedness for emergency management in dental settings, particularly those that use moderate sedation, is necessary. Certification in basic and advanced cardiac life support (ACLS) is mandatory for the team member providing sedation. All staff with patient contact must be certified in basic life support. A written emergency protocol should be in place containing specific protocols, a list of emergency equipment and drugs, and hospital transfer protocols. An up-to-date log to track the education, training, and certification of clinic staff should be maintained. Mock emergency drills should be practiced regularly to ensure that all staff members understand and perform their assigned duties during an actual anesthetic-related emergency.
For standardization of proper emergency treatment, development and implementation of treatment algorithms are essential. The American Dental Society of Anesthesiology, through the Anesthesia Research Foundation, has developed an application that uses individual patient information to form customized treatment algorithms.4 Treatment alternatives can then be retrieved and implemented in an emergency.
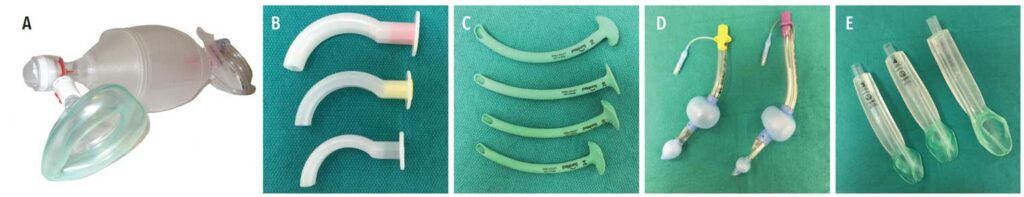
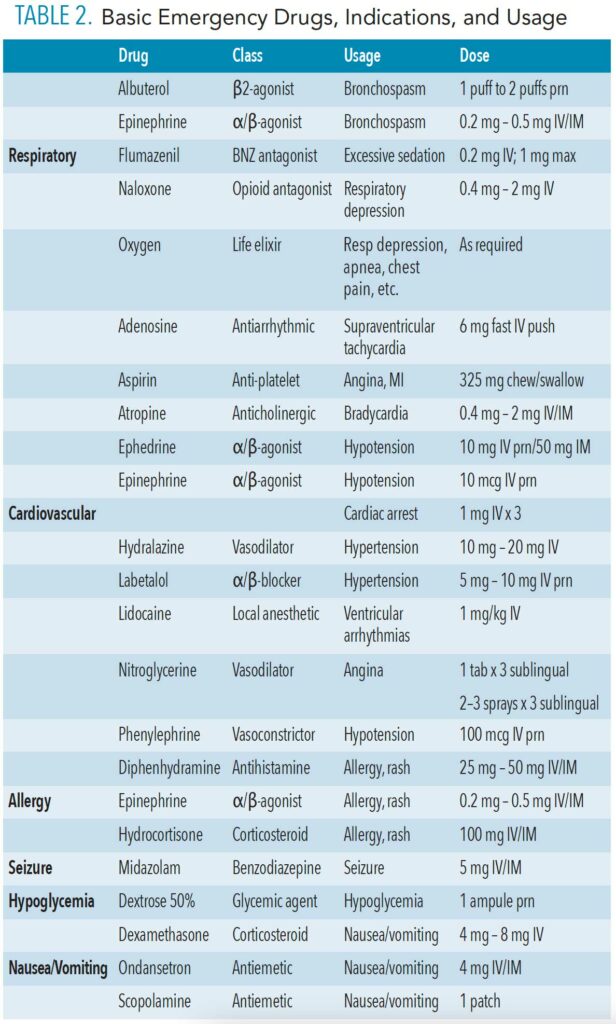
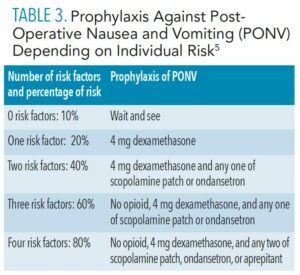
Management of perioperative emergencies requires an emergency drug kit and equipment that is specific for anesthetic-related emergencies. Table 1 and Table 2 (page 44) provide examples of a standard emergency kit and basic emergency drugs and their uses. Figure 1 shows essential airway management adjuncts.
Airway Complications
Obstruction. The primary cause of anesthetic-related morbidity and mortality is unidentified and/or untreated airway obstruction during anesthesia and sedation. Hence, maintaining the patient’s consciousness and capability to respond rationally to verbal commands ensures good airway patency. The patient’s ventilatory status and oxygenation must be continuously monitored using capnography and pulse oximetry. Loss of the capnographic waveform (an indicator of air movement in and out of the lungs) and/or oxygen desaturation is suggestive of airway obstruction.
The mere act of performing dental treatment on a sedated patient constitutes a threat to the airway. Airway manipulation during treatment can readily obstruct the airway. If improperly positioned, a mouth prop or tongue retractor may impinge upon the tongue and compromise the airway. During therapy, a handpiece with water spray, ultrasonic scaler, or irrigation is commonly used. A sedated patient may not be able to tolerate the excessive buildup of water or blood in the airway, which could lead to coughing, choking, laryngospasm, or bronchospasm.
When moderate sedation is the objective, deep sedation should be avoided at all costs. However, deep levels of sedation may develop inadvertently and must be immediately recognized and managed. The dentist must be adept at recognizing and managing the difficult airway. All office personnel should be trained to recognize and manage an obstructed airway, as this is the leading cause of anesthetic morbidity and mortality. Snoring, labored breathing with use of accessory chest and neck muscles (rocking-boat breathing), retraction of the suprasternal notch, tracheal tug, and loss of the end-tidal carbon dioxide waveform on the monitor are all signs of airway obstruction. Maneuvers such as the head tilt and chin lift, jaw thrust, and triple-airway maneuver are lifesaving if done quickly and efficiently. In the event these maneuvers are inadequate, the insertion of an adjunctive airway device is indicated (Figure 1). Nasal airways are available in different sizes and may be inserted through the nose in semiconscious or unconscious patients. The airway provides a patent air passage from the nose to the oropharynx and helps keep the tongue from obstructing the airway.
Oral airways are also available in various sizes and are inserted into the mouth of unconscious patients. These airways act to provide an air passage by moving the tongue from the posterior pharyngeal wall. In the event of nasal or oral airway failure, a supraglottic airway must be inserted to secure the airway. The i-gel Supraglottic Airway is recommended as an emergency airway due to its ease of insertion. Alternatively, the King LT airway device also provides a secure airway while protecting against gastric aspiration. Both of these devices are available in various sizes to accommodate a range of patients.
 Once the airway has been adequately addressed, the character of breathing should be immediately evaluated. The patient may be breathing adequately, or, more likely, either hypoventilating or completely apneic. Hypoventilation is recognized by assessing the rate and depth of breathing at chairside by matching the clinician’s own breathing to the patient’s. If the provider’s breathing seems to be inadequate, then the patient’s is too. Check the monitor for decreased oxygen saturation and elevated end-tidal carbon dioxide, as both are indicators of hypoventilation with a patent airway. If the patient can be aroused, encourage deep breathing and add supplemental oxygen if not already present.
Once the airway has been adequately addressed, the character of breathing should be immediately evaluated. The patient may be breathing adequately, or, more likely, either hypoventilating or completely apneic. Hypoventilation is recognized by assessing the rate and depth of breathing at chairside by matching the clinician’s own breathing to the patient’s. If the provider’s breathing seems to be inadequate, then the patient’s is too. Check the monitor for decreased oxygen saturation and elevated end-tidal carbon dioxide, as both are indicators of hypoventilation with a patent airway. If the patient can be aroused, encourage deep breathing and add supplemental oxygen if not already present.
If ventilation is still inadequate, respiration may be assisted by the application of a bag-valve-mask device with an oxygen flow of 10 L/min. Every fifth breath should be assisted by sealing the face mask and manually squeezing the bag. Apnea is recognized by the lack of breathing effort, oxygen desaturation, and absence of an end-tidal carbon dioxide waveform. Ventilation must be controlled again by using a bag-valve-mask with 100% oxygen at a rate of one breath every 5 seconds, or 12 times per minute. Once the airway has been secured and the patient is adequately ventilating and oxygenated, reversal agents, such as naloxone and flumazenil, may be given to restore consciousness.
Laryngospasm. In sedated patients, this response is a protective reflex occurring as a violent spasm of the vocal cords due to direct stimulation of the glottis by fluids, bleeding, or other foreign materials to prevent aspiration of these substances into the tracheobronchial tree. It is one of the most feared anesthetic-related mishaps. Laryngospasm results in a vigorous effort to breathe that can be accompanied by a high-pitched crowing sound. Oxygen desaturation and loss of capnographic waveform can also be observed. Patients with reactive airway disease, asthma, recent upper respiratory infections, or chronic obstructive pulmonary disease, as well as smokers (active or passive), are at increased risk for laryngospasm. Untreated laryngospasm can cause acute pulmonary edema, hypoxemia, or cardiac arrest.
Laryngospasm can be avoided by minimizing bleeding, secretions, water spray (from ultrasonic scalers and handpieces), appropriate suctioning by a dental assistant, and use of a throat screen. Maintaining patient consciousness is the most efficient method of preventing laryngospasm during moderate sedation.
Application of sustained positive pressure with 100% oxygen through a bag-valve-mask device is usually adequate to overcome laryngospasm. This should be followed by reversal of the sedative medications.
 Bronchospasm. Resulting from rapid bronchial airway constriction, bronchospasm is an acute emergent event manifesting as dyspnea, coughing, wheezing, hypoxemia, and hypercarbia (ie, too much CO2 in the blood; also known as hypercapnia). If left untreated, it can become life-threatening. Patients with a history of reactive airway disease, such as chronic bronchitis, emphysema, or asthma, as well as smokers, are predisposed to bronchospasm. It can also be caused by exacerbation of asthma, prolonged airway obstruction, laryngospasm, or debris in the airway. Capnographic examination also aids in the diagnosis of bronchospasm. A classic “shark fin” ETCO2 waveform is suggestive of lower airway obstruction.
Bronchospasm. Resulting from rapid bronchial airway constriction, bronchospasm is an acute emergent event manifesting as dyspnea, coughing, wheezing, hypoxemia, and hypercarbia (ie, too much CO2 in the blood; also known as hypercapnia). If left untreated, it can become life-threatening. Patients with a history of reactive airway disease, such as chronic bronchitis, emphysema, or asthma, as well as smokers, are predisposed to bronchospasm. It can also be caused by exacerbation of asthma, prolonged airway obstruction, laryngospasm, or debris in the airway. Capnographic examination also aids in the diagnosis of bronchospasm. A classic “shark fin” ETCO2 waveform is suggestive of lower airway obstruction.
Bronchodilation is the mainstay of treatment for bronchospasm. Initially, an albuterol inhaler can be used for bronchodilation. Epinephrine should be administered if albuterol proves inadequate. It can be administered intravenously at a dose of 0.2 mg to 0.5 mg or can be up-titrated by 10 µg until effective. Owing to its cardiac-stimulating property, epinephrine should be carefully titrated in patients with cardiovascular disease.
Emesis/Aspiration. Post-operative nausea and vomiting (PONV) are prevalent and usually self-limiting complications after surgery that may persist for 2 days to 3 days. They lead to marked patient dissatisfaction. A high risk of aspirating gastric contents exists if PONV occur during sedation. Therefore, identification of patients at risk before treatment is helpful for the application of preventive measures.5 Common risk factors for PONV include:
- Female gender
- History of PONV or motion sickness
- Nonsmoker
- General vs regional anesthesia
- Use of volatile anesthetics or nitrous oxide
- Opioid use
- Duration of anesthesia
- Dental surgery (swallowing of blood)
After determining the patient’s risk, PONV can be minimized with the use of prophylactic drug therapy (Table 3). The drugs used for prophylaxis are also commonly used for the treatment of PONV; these include dexamethasone, ondansetron, scopolamine patch, and aprepitant.
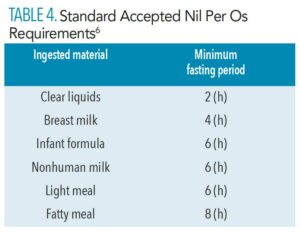
There is a risk of aspiration or inhalation of gastric contents into the lungs in case of emesis during moderate sedation. It can lead to pneumonia or acute chemical pneumonitis. Hence, the risk of aspiration during procedures can be reduced by strictly following the American Society of Anesthesiologists fasting nil per os (NPO) guidelines (Table 4).6
 With regard to managing emesis/aspiration complications, patients with chronic impairment of airway reflexes, such as those with stroke, seizure disorders, multiple sclerosis, cerebral palsy, Parkinsonism, Down syndrome, alcoholism, or amyotrophic lateral sclerosis, are prone to develop aspiration pneumonia. Emesis and aspiration during sedation can cause chemical burns to the tracheobronchial tree (Mendelson syndrome) that result in coughing, wheezing, respiratory distress, and pulmonary edema. This condition is considered life-threatening and needs immediate maintenance of the airway, clearance of secretions, oxygen supplementation, and immediate transfer to a hospital for tracheal intubation.
With regard to managing emesis/aspiration complications, patients with chronic impairment of airway reflexes, such as those with stroke, seizure disorders, multiple sclerosis, cerebral palsy, Parkinsonism, Down syndrome, alcoholism, or amyotrophic lateral sclerosis, are prone to develop aspiration pneumonia. Emesis and aspiration during sedation can cause chemical burns to the tracheobronchial tree (Mendelson syndrome) that result in coughing, wheezing, respiratory distress, and pulmonary edema. This condition is considered life-threatening and needs immediate maintenance of the airway, clearance of secretions, oxygen supplementation, and immediate transfer to a hospital for tracheal intubation.
Aspiration pneumonia is a gradually developing disease resulting from small amounts of gastric aspiration or inhalation of aerosolized water spray. It has nonspecific symptoms, including dyspnea on exertion, shortness of breath, and hypoxemia with a subacute onset. Clinicians should strive to minimize or eliminate water spray from ultrasonic scalers and handpieces while treating vulnerable patients, as the resultant aerosols can easily be inhaled into the lungs and lead to a bacterial pneumonia. This infection requires antibiotic therapy and hospitalization, if necessary.
Cardiovascular Complications
Hypertension. Among those with preexisting cardiovascular conditions, particularly hypertension, appropriate patient evaluation and optimization before treatment are essential to prevent cardiac mishaps, such as myocardial ischemia and infarction, or stroke. Stress from dental therapy can induce a cardiovascular stress response, with increased heart rate, blood pressure, and cardiac output. This response can be controlled by moderate sedation with careful intraoperative monitoring of heart rate and blood pressure. Emergency measures for optimal control should be readily available to prevent a catastrophe.
Cardiac stress can be assessed by calculating the rate pressure product (RPP); that is, the product of the heart rate times the systolic blood pressure. A relatively normal RPP is less than 12,000, indicating very little cardiac stress. For RPPs greater than 20,000, a significant cardiac stress exists that can require treatment. Autoregulatory systems function well in individuals with preexisting heart conditions or hypertension when their blood pressure values are as close to baseline as possible. Hence, maintenance of blood pressure within a 20% range above and below the baseline of mean arterial pressure (MAP) is necessary after optimization of blood pressure to ensure adequate organ perfusion.
 Management of patients with blood pressure fluctuation within the acceptable range can be done intraoperatively. In case of a rise in blood pressure above the acceptable range, definitive treatment should be initiated after ruling out the underlying etiology. Bladder distension is usually overlooked as a cause of hypertension. Hence, intraoperative hypertension can be avoided by asking patients to empty the bladder before inducing sedation.
Management of patients with blood pressure fluctuation within the acceptable range can be done intraoperatively. In case of a rise in blood pressure above the acceptable range, definitive treatment should be initiated after ruling out the underlying etiology. Bladder distension is usually overlooked as a cause of hypertension. Hence, intraoperative hypertension can be avoided by asking patients to empty the bladder before inducing sedation.
Pain during treatment can result in hypertension in controlled hypertensive patients. Therefore, the use of local anesthesia is advocated to efficiently manage pain to prevent a hypertensive urgency. In fact, profound local anesthesia is key to successful moderate sedation in all cases. Addition of intravenous opioid medication does not control operative pain and can lead to respiratory depression. Use of vasoconstrictors should be minimized in patients with cardiovascular disease, and continuous monitoring is indicated.
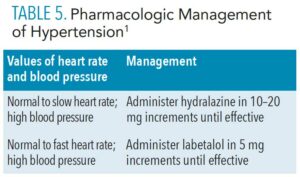
Pharmacologic intervention is required if the blood pressure remains above the acceptable level even after addressing the preexisting causes of hypertension. Management depends on heart rate and blood pressure values (Table 5).
Hypotension. During sedation procedures, profound hypotension can be more disastrous than hypertension. Marked hypotension can stop vital organ perfusion and result in cerebral, ocular, myocardial, and renal ischemia, and renal failure, particularly in patients with coronary artery disease, aortic stenosis, valvular disorders, or heart failure. Hence, maintenance of MAP as close to the baseline value as possible is essential. If MAP drops to less than 80% of the baseline value, corrective treatment should be initiated immediately. In no case should the patient’s MAP be allowed to fall below 65 mmHg. Hypotension during sedation is usually associated with patient response to sedatives, drug interactions, or dehydration.
In terms of preventive measures, following strict fasting guidelines can increase the risk of hypovolemia and subsequent hypotension during sedation. In such cases, preoperative carbohydrate loading approximately 2 hours before the procedure can provide effective hydration, decrease the risk of PONV, and facilitate recovery. A sugar and electrolyte solution is recommended.
Reduced cardiac output produces hypotension. This can be corrected by increasing the venous return to the heart. To increase venous return, the patient is given a rapid intravenous fluid bolus, along with adjustment of the dental chair to a Trendelenburg position (ie, head down, feet up).
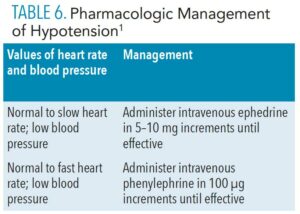
Vasoactive drugs are required when the aforementioned maneuvers prove inadequate for correcting hypotension. Drug administration will depend on the clinician’s evaluation of heart rate and blood pressure (Table 6, page 45).
Blood pressure fluctuations—either too high or too low—are the most common cardiovascular emergent situations encountered during sedation procedures. Although much less likely, patients may experience significant cardiac dysrhythmias, acute coronary syndromes, or stroke during treatment. These conditions are best managed through the application of ACLS protocols.
Miscellaneous Complications
Local Anesthetic Toxicity. Local anesthetic toxicity is a commonly underestimated complication and can have serious adverse effects. Older adults and patients with cardiac issues, such as conduction defects, coronary artery disease, ischemia, or heart failure, are prone to local anesthetic toxicity. Children have a high risk of developing local anesthetic overdose. Hence, calculation of maximum recommended dose of local anesthetic for each patient is essential. The dose administered should be noted in the patient record.
The cardiovascular system (CVS) and central nervous system (CNS) are affected by local anesthetics in a dose-dependent manner. Clinically, CNS toxicity manifests as an initial excitatory response with light-headedness, dizziness, anxiety, confusion, visual and auditory disturbances, involuntary muscle movement, and seizure activity, followed by depressive effects characterized by drowsiness, slurred speech, unconsciousness, and respiratory depression or arrest. The effects of CVS include massive vasodilation, severe myocardial depression, and cardiac arrest. Due to the varied signs of CNS and CVS toxicity, it is essential to suspect local anesthetic toxicity in case of changes in blood pressure, heart rhythm, or mental status. As children are prone to local anesthetic toxicity and overdose, the American Academy of Pediatric Dentistry recommends limiting the maximum dose of lidocaine to 4.4 mg/kg (2 mg/lb) and articaine to 7 mg/kg (3.2 mg/lb).7
Local anesthetic toxicity can mostly be managed with supportive therapy. Intralipid infusion is therapeutic if profound CVS or CNS depression occurs. It is a purified soybean oil fat emulsion that produces a “lipid sink” and decreases the serum concentration of the local anesthetic when administered intravenously. This reverses the toxic reaction by rapidly reducing the local anesthetic blood levels.
 Delayed Awakening and Readiness for Discharge. Drug overdose is considered the most probable cause of delayed awakening following sedation. Pharmacological reversal of sedation may be necessary when arousal becomes difficult. The patient should be monitored for signs of re-sedation for an hour after the administration of reversal agents. Significant drug interactions can also contribute to difficulty in arousal. In such cases, the patient’s medication list should be assessed for possible interactions between those drugs and sedative agents.
Delayed Awakening and Readiness for Discharge. Drug overdose is considered the most probable cause of delayed awakening following sedation. Pharmacological reversal of sedation may be necessary when arousal becomes difficult. The patient should be monitored for signs of re-sedation for an hour after the administration of reversal agents. Significant drug interactions can also contribute to difficulty in arousal. In such cases, the patient’s medication list should be assessed for possible interactions between those drugs and sedative agents.
If drug overdose has been excluded as a cause for delayed awakening, the effect of the five “H’s” (hypotension, hypothermia, hypercarbia, hypoxemia, and hypoglycemia) should be evaluated. Hypoglycemia should be considered after ruling out hypotension, hypothermia, hypercarbia, and hypoxemia by routine monitoring. A glucometer can be used to measure blood glucose levels for diagnosis. The patient should also be evaluated for stroke.
The time to discharge should be determined by evaluating the patient’s ability to independently maintain protective reflexes, regain coordination, ambulate with minimal assistance, and be safely transferred to the care of responsible personnel. To ensure patient safety, a quantitative discharge assessment tool—such as the post-anesthetic discharge scoring system that standardizes post-sedation home readiness—should be utilized.8
Conclusion
Emergency preparedness is key in preventing and managing emergent situations during moderate sedation. With recent advances, patients with debilitating diseases may become medically stable enough to undergo dental treatment. However, these patients may have an increased risk for experiencing perioperative emergencies. In all situations, and particularly when medical complexities exist, dental teams should be prepared and equipped to identify and manage perioperative emergencies.
References
- Giovannitti JA. Moderate Sedation and Emergency Medicine for Periodontists. Cham, Switzerland: Springer; 2020.
- Becker ED, Haas DA. Recognition and management of complications during moderate and deep sedation, part 1: respiratory considerations. Anesthesia Progress. 2011;58:82–92.
- Becker ED, Haas DA. Recognition and management of complications during moderate and deep sedation, part 2: cardiovascular considerations. Anesthesia Progress. 2011;58:126–138.
- Anesthesia Research Foundation. Ten Minutes Saves a Life! Available at: adsa-arf.org/tenminutes. Accessed October 12, 2021.
- Apfel CC, Laara E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology. 1999;91:693–700.
- Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: a report by the American Society of Anesthesiologist Task Force on Preoperative Fasting. Anesthesiology. 1999;90:896–905.
- American Academy of Pediatric Dentistry. Use of local anesthesia for pediatric dental patients. The Reference Manual of Pediatric Dentistry. 2020;318–323.
- Chung F, Chan VW, Ong D. A post-anesthetic discharge scoring system for home readiness after ambulatory surgery. J Clin Anesth. 1995;7:500–506.
From Dimensions of Dental Hygiene. January 2022;20(1):42-46.