 FIGURE 1A COURTESY CADE DAUGHENBAUGH, DDS; FIGURE 1B COURTESY DAVID BULOT, DDS, MD
FIGURE 1A COURTESY CADE DAUGHENBAUGH, DDS; FIGURE 1B COURTESY DAVID BULOT, DDS, MD
Detecting Premalignant and Malignant White Lesions
Differentiating between malignant/ premalignant oral mucosal lesions and benign white lesions is key to ensuring positive health outcomes.
This course was published in the November 2020 issue and expires November 2023. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Describe various etiologies and classifications for premalignant and malignant oral mucosal lesions, as well as prevalence rates for malignant transformation.
- Explain strategies for examination and diagnosis of white oral mucosal lesions.
- Discuss approaches to managing premalignant and malignant oral mucosal lesions.
This is the concluding installment of a two-part series. Appearing in October 2020, Part 1 (available at dimensionsofdentalhygiene.com) offers clinical insights into diagnosing benign white oral mucosal lesions.
Oral health professionals are likely to identify white oral mucosal lesions in their patients and face the challenge of distinguishing benign lesions from potentially malignant disorders. Accurately identifying these lesions is an important aspect of daily dental and dental hygiene practice, and providers should understand that some features are more ominous than others. White lesions may develop from a variety of etiologic pathways, including, but not limited to, inflammatory, traumatic, and neoplastic. This article focuses on white lesions that are most likely premalignant or malignant to help oral health professionals gain confidence in recognizing these conditions. It will also offer suggestions for care once such lesions are identified. While the first article in this series, “Diagnostic Tips for Benign White Oral Mucosal Lesions” (available at dimensionsofdentalhygiene.com), covered benign lesions, this concluding installment will focus on white lesions that are malignant or premalignant.
POTENTIALLY MALIGNANT DISORDERS
Leukoplakia is a clinical term only, meaning it is not a definitive diagnosis. A biopsy is mandatory to determine what the white plaque represents. When biopsied, the microscopic diagnosis may be hyperkeratosis; epithelial atypia; mild, moderate, or severe epithelial dysplasia, carcinoma in situ; or squamous cell carcinoma (SCC). Epithelial dysplasia is present in 16% to 39% of oral leukoplakia, and SCC is present in approximately 3% of oral leukoplakia, further supporting the importance of biopsy to obtain a definitive diagnosis.1,2
The term leukoplakia itself can be confusing. Leuko means white, and plakia means plaque, but the definition has evolved over the years. The most recent iteration being a “white plaque of questionable risk, having excluded (other) known diseases or disorders that carry no increased risk for cancer,” which was proposed at a World Health Organization workshop in 2005.3 What does this mean to the practitioner? Oral leukoplakia is a white plaque that cannot be wiped off, cannot be diagnosed clinically as another definable entity, and has a risk to develop into cancer. It is therefore considered a potentially malignant disorder, meaning it has an increased risk for malignant transformation.4
Rates of oral leukoplakia undergoing malignant transformation vary broadly in the literature. A 2019 systematic review and meta-analysis revealed 9.5% of oral leukoplakia was found to evolve into cancer, with a 1.56% annual malignant transformation rate.5 Risk factors associated with malignant transformation include presence of epithelial dysplasia on biopsy, female gender, older age, extensive lesion duration, absence of tobacco and/or alcohol use, lateral tongue or floor of mouth lesions, size greater than 2 cm, nonhomogeneous clinical appearance, and previous head or neck cancer.1,4
 The prevalence of oral leukoplakia is approximately 2% to 3% worldwide.6,7 It has a male predominance and is usually identified in the fifth decade or after. The most common sites include the lateral and ventral tongue, floor of mouth, buccal mucosa, hard and soft palate, and alveolar/gingival mucosa.6,8,9 Risk factors associated with oral leukoplakia include tobacco or betel quid use and alcohol consumption.10 This emphasizes the importance of reviewing the complete medical and social history for each patient.10 Suspicion of potentially malignant disorders should be elevated if a patient reports these types of behaviors.
The prevalence of oral leukoplakia is approximately 2% to 3% worldwide.6,7 It has a male predominance and is usually identified in the fifth decade or after. The most common sites include the lateral and ventral tongue, floor of mouth, buccal mucosa, hard and soft palate, and alveolar/gingival mucosa.6,8,9 Risk factors associated with oral leukoplakia include tobacco or betel quid use and alcohol consumption.10 This emphasizes the importance of reviewing the complete medical and social history for each patient.10 Suspicion of potentially malignant disorders should be elevated if a patient reports these types of behaviors.
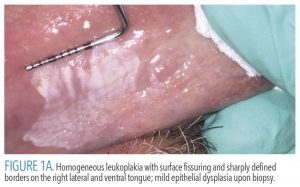
Oral leukoplakias are solitary lesions that frequently will have sharply defined borders in at least one area, which raises concern for a potentially malignant disorder.11 This condition is divided into two categories: homogenous and nonhomogeneous.3 Homogeneous lesions are evenly white, predominantly thin, flat, possibly with fine fissures, and have sharply defined borders (Figure 1A). Nonhomogenous lesions exhibit variations, including thickenings (either verrucous or nodular; Figure 1B) or speckled erythematous (red) areas. Nonhomogenous lesions are considered higher risk for dysplasia or malignancy.3,10,12
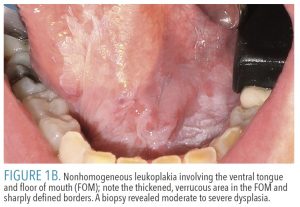 When examining a leukoplakia, an accurate measurement should be taken (Figure 1A), the borders assessed, and the lesion palpated for induration (firmness) of the underlying tissue and to see if the patient perceives any discomfort. High-quality photographs should be taken for documentation purposes and to facilitate communication with referrals. Photos can also be a useful tool for explaining findings to patients, allowing them to better visualize the lesion and understand what has been identified. Clinicians may opt to reassess in 2 weeks and if no changes are detectable, a biopsy is indicated. While some general dentists perform biopsies, if a lesion is concerning for a potentially malignant disorder, a referral for assessment and biopsy by an oral and maxillofacial surgeon is recommended. If the patient has risk factors, such as tobacco or alcohol use, cessation should be encouraged.10,13
When examining a leukoplakia, an accurate measurement should be taken (Figure 1A), the borders assessed, and the lesion palpated for induration (firmness) of the underlying tissue and to see if the patient perceives any discomfort. High-quality photographs should be taken for documentation purposes and to facilitate communication with referrals. Photos can also be a useful tool for explaining findings to patients, allowing them to better visualize the lesion and understand what has been identified. Clinicians may opt to reassess in 2 weeks and if no changes are detectable, a biopsy is indicated. While some general dentists perform biopsies, if a lesion is concerning for a potentially malignant disorder, a referral for assessment and biopsy by an oral and maxillofacial surgeon is recommended. If the patient has risk factors, such as tobacco or alcohol use, cessation should be encouraged.10,13
The management of oral leukoplakia is dependent on definitive diagnosis. Complete excision of epithelial dysplasia, carcinoma in situ or SCC is recommended. Patients with one potentially malignant oral disorder are at increased risk for developing an additional lesion, or recurrence at the site of the primary lesion. Recurrence rates of oral leukoplakia following treatment are 5% to 10%.14 There are no clear guidelines for follow-up after excision; however, many sources recommend follow-up every 3 months to 6 months for the patient’s lifetime.4,9,10,14
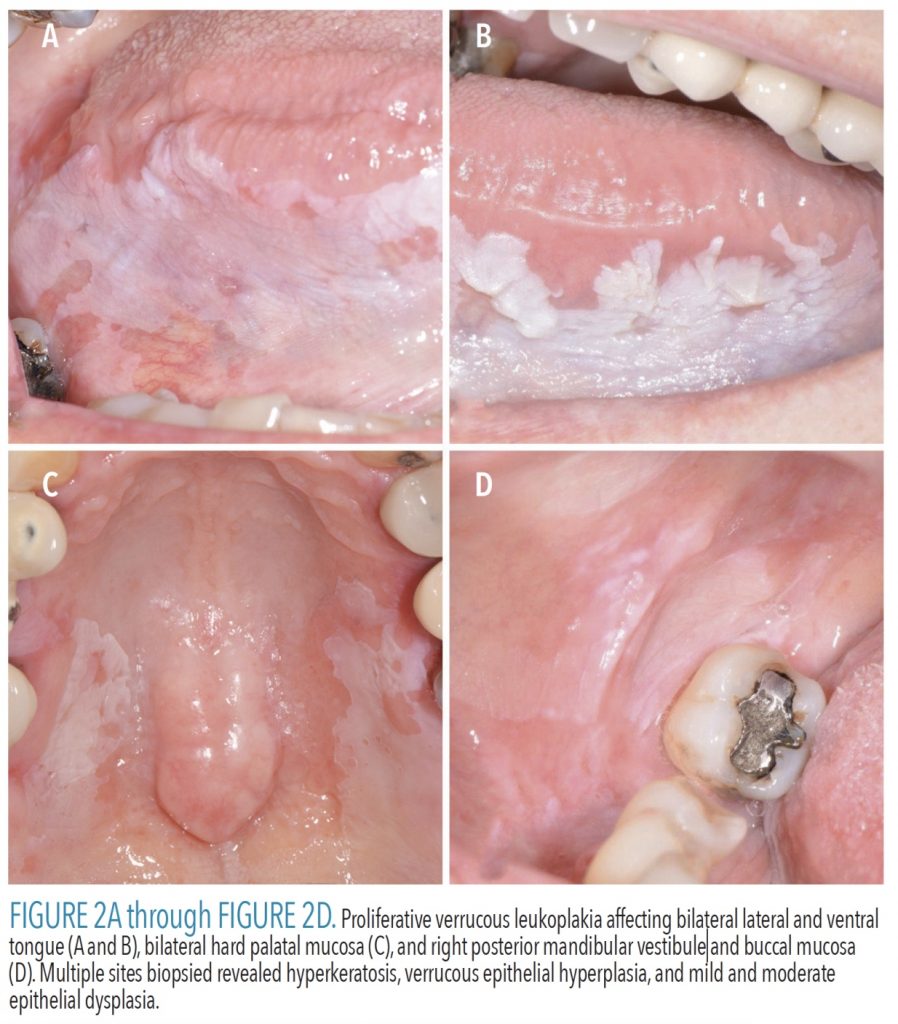
Proliferative verrucous leukoplakia (PVL) is a potentially malignant disorder initially reported by Hansen et al15 in 1985 as a “slow-growing, persistent and irreversible” process with a very high malignant transformation rate ranging from 70% to 100%.15–17 As this condition is so newly recognized, prevalence data are not currently available.17 No known etiology has been identified.17 Patients are more commonly nonsmokers (< 30% report smoking) and female (2.5–5:1).17 Patients may initially present with a solitary white plaque that gradually proliferates; frequently, multiple additional lesions will develop and become widespread. Commonly affected sites include gingiva/alveolar mucosa, buccal mucosa, and ventral tongue.17 Criteria for diagnosis continue to evolve. In 2018, Villa et al17 suggested PVL be renamed “proliferative leukoplakia” and proposed three clinical criteria to aid in diagnosis: 1) smooth, fissured, verrucous white or red lesions, with or without ulceration; 2) multifocal, noncontiguous lesions, a single large lesion > 4 cm, or a single > 3-cm lesion involving contiguous sites; or 3) lesions that progress in size and/or become multifocal. If a single leukoplakia is identified, clinicians should closely reexamine the entire oral cavity looking for additional lesions, with the understanding that early recognition and diagnosis of PVL may lead to earlier detection of malignant transformation and therefore improved prognosis.17
Upon clinical examination, lesions of PVL will appear remarkably similar to oral leukoplakia, with the important addition of larger size, multiple sites and progression (Figure 2A, page 41, through Figure 3B). The clinician must recognize these features likely establish a more serious diagnosis. Patients will need multiple biopsies of affected sites and the pathologist must be informed of the clinical presentation. Inclusion of photographs with submission of biopsy specimens to an oral and maxillofacial pathologist is strongly encouraged, not just for this condition, but for all oral lesions. Histopathologically, PVL exhibits a spectrum of features, including hyperkeratosis, verrucous epithelial hyperplasia, epithelial atypia, epithelial dysplasia, carcinoma in situ, verrucous carcinoma, and SCC. The diagnosis of PVL ultimately requires clinicopathologic correlation, meaning correlating the clinical findings with the microscopic diagnosis to determine if the patient fulfills the clinical diagnostic criteria of PVL.
Given the progressive nature of PVL, patients will likely need additional and repeat biopsies (particularly of areas that become verrucous/nodular) to monitor for transition to dysplasia and/or SCC.16,17 Treating PVL is extremely challenging, with surgical excision considered the therapy of choice. Even with excision, recurrence rates reach 71.2%.16 No currently available treatment has been shown to reduce the risk of transformation to cancer.10,15,17 In 2018, Villa et al17 proposed a thorough management strategy for PVL patients, including: 1) photography at every visit; 2) follow-up every 3 months to 6 months, with periodic biopsies as new lesions develop or existing ones change; 3) monitoring of lesions on biopsy shown to have no dysplasia; 4) preferred removal of lesions with mild to moderate dysplasia, but if not clinically possible due to extent or location of the lesion, monitor closely; and 5) surgical removal of lesions that are severe dysplasia, carcinoma in situ, verrucous carcinoma or SCC.
![FIGURE 3A and FIGURE 3B. Proliferative verrucous leukoplakia affecting buccal, facial, and palatal gingiva from teeth #3 to 11 (A); biopsy indicated squamous cell carcinoma of palatal tissues (B).]() MALIGNANT DISORDERS
MALIGNANT DISORDERS
Representing 90% of cancers affecting the oral cavity and oropharynx, SCC accounts for 3% of all cancers in the United States.1,18 It is estimated that 53,260 cases and 10,750 deaths will occur in 2020.18 Incidence is higher in men (17.2 males:6.4 females/100,000 people), and it is most commonly diagnosed between ages 55 and 64 (median age 63).18 Understanding the anatomic distinction between oral cavity SCC and oropharyngeal SCC is important. The oropharynx is composed of the soft palate, posterior one-third (base) of tongue, palatine tonsils, palatoglossal folds, valleculae, and posterior pharyngeal wall. The majority of oropharyngeal SCCs develop in the tonsillar region and base of tongue.1 The oral cavity consists of all oral sites anterior to those just listed, with cancers in the Western world most commonly affecting the lateral and ventral tongue and floor of mouth. In geographic areas where betel quid is commonly used, tongue and buccal mucosa are the most common sites.1 Known risk factors for oral cavity SCC are the same as oral leukoplakia: smoked tobacco, smokeless tobacco, heavy alcohol consumption, and use of betel quid.1,19,20
 Oropharyngeal SCC risk factors include smoked tobacco, heavy alcohol use, and high-risk human papillomavirus (HPV) infection.21 For both sites, combined use of smoked tobacco and alcohol consumption exhibits a synergistic effect, increasing the relative risk of cancer development by as much as 15 in heavy users.1 Only a small fraction of oral cavity SCCs have been shown to be HPV-related, and routine testing for HPV infection is not recommended for cancers of the oral cavity.21,22 As with premalignant lesions, if a patient has risk factors—such as tobacco or alcohol use—cessation should be encouraged.10,13
Oropharyngeal SCC risk factors include smoked tobacco, heavy alcohol use, and high-risk human papillomavirus (HPV) infection.21 For both sites, combined use of smoked tobacco and alcohol consumption exhibits a synergistic effect, increasing the relative risk of cancer development by as much as 15 in heavy users.1 Only a small fraction of oral cavity SCCs have been shown to be HPV-related, and routine testing for HPV infection is not recommended for cancers of the oral cavity.21,22 As with premalignant lesions, if a patient has risk factors—such as tobacco or alcohol use—cessation should be encouraged.10,13
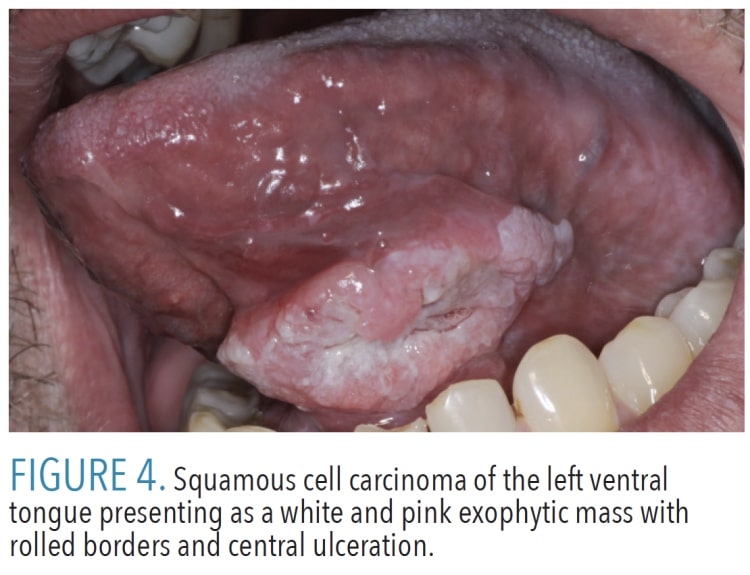
Clinically, oral cavity SCC may be variable in appearance, presenting with any of the following features: leukoplakia, erythroplakia (red plaque), erythroleukoplakia (mixed red and white plaque), exophytic proliferations or masses (verrucous or nodular), ulceration, rolled borders, and induration of underlying tissues (Figure 4). Other important clinical findings include pain and altered neurologic function, including numbness and decreased mobility; although, many cancers present without symptoms other than the presence of a clinically detectable lesion. When assessing lesions, clinicians cannot exclude cancer as a possibility even if the patient has no symptoms related to the lesion.
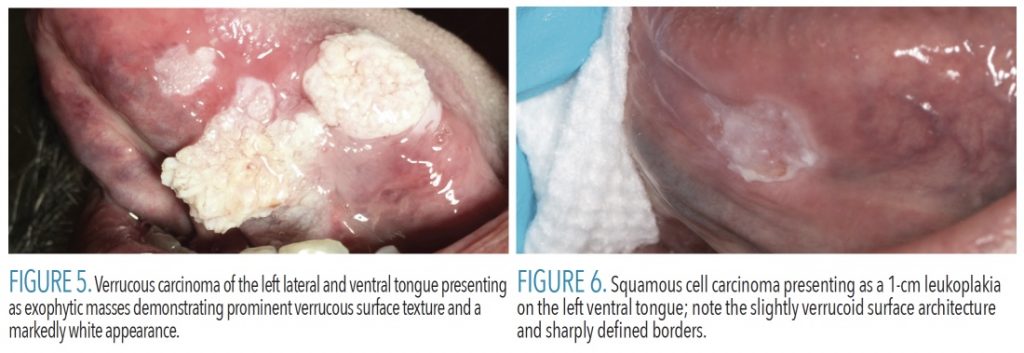
Another entity that may be included in the differential diagnosis is verrucous carcinoma, which is considered to be a low-grade variant of oral cavity SCC. Verrucous carcinomas typically present as markedly exophytic, verrucous, broad-based, firm, and with white proliferations usually lacking ulceration23,24 (Figure 5). Clinicians need to remember that ~3% of oral leukoplakia represent oral cavity SCC, therefore, even small white lesions will require follow-up and biopsy (Figure 6). Oropharyngeal SCC may present as an irregular red or ulcerated mass, or it may be clinically undetectable.1 Neck mass, sore throat, and difficulty swallowing are common complaints from patients with HPV-positive oropharyngeal SCC.1
When a suspected oral cavity SCC or oropharyngeal SCC is recognized during head and neck examination, as with leukoplakia and PVL, photographs should be taken and the patient referred to an experienced oral and maxillofacial surgeon or head and neck surgeon. Scheduling the referral appointment prior to the patient’s departure from the office is strongly recommended. Patients should be informed of the clinical suspicion so they understand the importance of following through with the next step in the diagnostic process, which is biopsy. Treatment of oral cavity SCC and oropharyngeal SCC may include surgery, neck dissection, radiation, and chemotherapy. Prognosis is dependent on staging at time of diagnosis, with the current 5-year relative survival rate of all stages reported as 66.2%.18 When this 5-year percentage is broken down by stage—localized disease (85.1%), spread to regional lymph nodes (66.8%), and distant metastasis (40.1%)—the difference early diagnosis can make is evident.18 Patients require lifelong follow-up every 3 months to 6 months. This follow-up will likely be a collaborative effort on the part of the patient with the treating oncologic surgeon, as well as the general dentist providing regular examinations.
CONCLUSION
General dentists and dental hygienists play a critical role in detection of suspicious oral lesions, and providing appropriate treatment or directing patients to a specialist better suited for necessary care. The ability to differentiate white lesions that are most likely benign from those that are worrisome for dysplasia or malignancy can be a challenge for even the most experienced clinicians. Any lesion thought to be a potentially malignant disorder should be referred to an oral and maxillofacial surgeon for biopsy to obtain a definitive diagnosis. Early diagnosis is critical to improving clinical outcomes and survival for patients with premalignant or malignant oral mucosal lesions.
References
- Chi AC, Day TA, Neville BW. Oral cavity and oropharyngeal squamous cell carcinoma — an update. CA Cancer J Clin. 2015;65:401–421.
- Waldron CA, Shafer WG. Leukoplakia revisited. A clinicopathologic study 3256 oral leukoplakias. Cancer. 1975;36:1386–1392.
- Warnakulasuriya S, Johnson NW, van der Waal I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med. 2007;36:575–580.
- van der Waal I. Potentially malignant disorders of the oral and oropharyngeal mucosa; terminology, classification and present concepts of management. Oral Oncol. 2009;45:317–323.
- Iocca O, Sollecito TP, Alawi F, et al. Potentially malignant disorders of the oral cavity and oral dysplasia: A systematic review and meta-analysis of malignant transformation rate by subtype. Head Neck. 2020;43:539–555.
- Napier SS, Speight PM. Natural history of potentially malignant oral lesions and conditions: an overview of the literature. J Oral Pathol Med. 2008;37:1–10.
- Petti S. Pooled estimate of world leukoplakia prevalence: a systematic review. Oral Oncol. 2003;39:770–780.
- Jones KB, Jordan R. White lesions in the oral cavity: clinical presentation, diagnosis, and treatment. Semin Cutan Med Surg. 2015;34:161–170.
- Wetzel SL, Wollenberg J. Oral potentially malignant disorders. Dent Clin North Am. 2020;64:25–37.
- Staines K, Rogers H. Oral leukoplakia and proliferative verrucous leukoplakia: a review for dental practitioners. Br Dent J. 2017;223:655–661.
- Villa A, Woo SB. Leukoplakia — a diagnostic and management algorithm. J Oral Maxillofac Surg. 2017;75:723–734.
- Woo SB. Oral epithelial dysplasia and premalignancy. Head Neck Pathol. 2019;13:423–439.
- Warnakulasuriya S. White, red, and mixed lesions of oral mucosa: a clinicopathologic approach to diagnosis. Periodontol 2000. 2019;80:89–104.
- van der Waal I. Oral potentially malignant disorders: is malignant transformation predictable and preventable? Med Oral Patol Oral Cir Bucal. 2014;19:e386–e390.
- Hansen LS, Olson JA, Silverman S. Proliferative verrucous leukoplakia. A long-term study of thirty patients. Oral Surg Oral Med Oral Pathol. 1985;60:285–298.
- Abadie WM, Partington EJ, Fowler CB, Schmalbach CE. Optimal management of proliferative verrucous leukoplakia: a systematic review of the literature. Otolaryngol Head Neck Surg. 2015;153:504–511.
- Villa A, Menon RS, Kerr AR, et al. Proliferative leukoplakia: proposed new clinical diagnostic criteria. Oral Dis. 2018;24:749–760.
- National Institutes of Health. National Cancer Institute. SEER Cancer Statistics Review (CSR) 1975–2017. Available at: https://seer.cancer.gov/csr/1975_2017/. Acccessed July 8, 2020.
- Mortazavi H, Safi Y, Baharvand M, Jafari S, Anbari F, Rahmani S. Oral white lesions: an updated clinical diagnostic decision tree. Dent J (Basel). 2019;7:15.
- Bewley AF, Farwell DG. Oral leukoplakia and oral cavity squamous cell carcinoma. Clin Dermatol. 2017;35:461–467.
- Mirghani H, Amen F, Moreau F, Lacau St Guily J. Do high-risk human papillomaviruses cause oral cavity squamous cell carcinoma? Oral Oncol. 2015;51:229–236.
- Muller S. Update from the 4th edition of the World Health Organization of head and neck tumours: tumours of the oral cavity and mobile tongue. Head Neck Pathol. 2017;11:33–40.
- Thompson LD. Verrucous squamous cell carcinoma. Ear Nose Throat J. 2019:145561319871712.
- Walvekar RR, Chaukar DA, Deshpande MS, et al. Verrucous carcinoma of the oral cavity: a clinical and pathological study of 101 cases. Oral Oncol. 2009;45:47–51.
From Dimensions of Dental Hygiene. November 2020;18(10):40-43.