Detecting Gingival Cancer
Oral health professionals play a crucial role in the early detection and post-therapy management of this disease.
This course was published in the December 2017 issue and expires December 2020. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Discuss the incidence of gingival squamous cell carcinoma (SCC), recurrence, and the characteristics that make diagnosis challenging.
- Explain the oral health professional’s role in identifying and treating patients with gingival SCC.
- List imaging modalities and treatments used in managing gingival SCC.
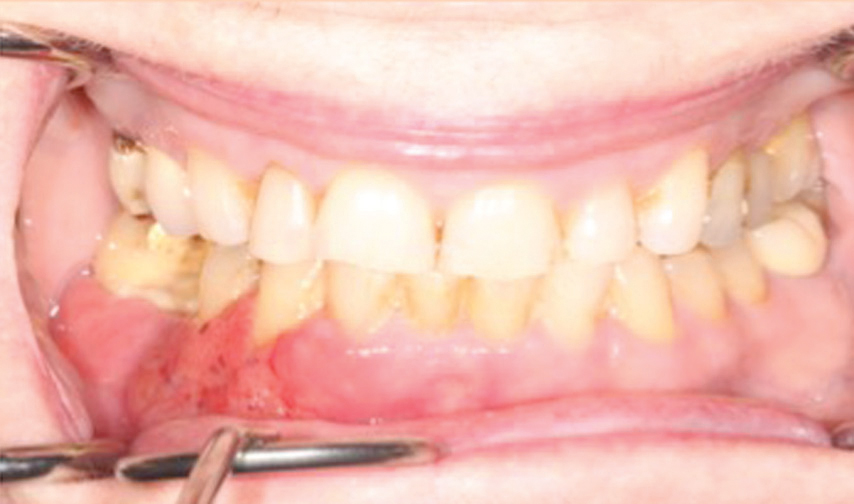
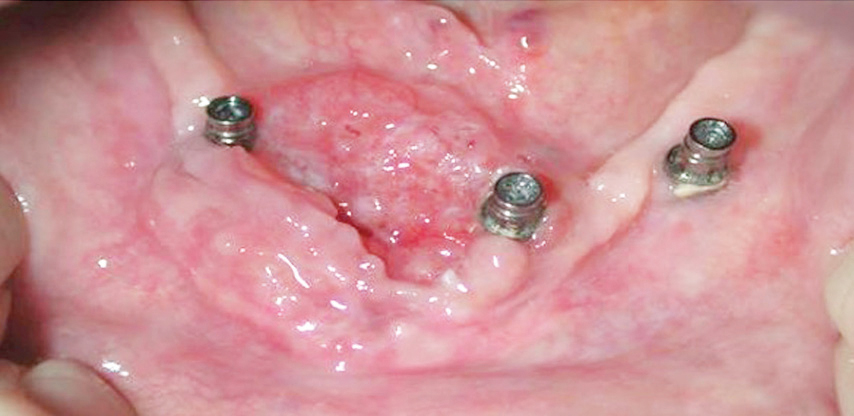
An initial diagnosis of gingival SCC can be difficult to establish, as early lesions often present with an appearance similar to inflammatory periodontal conditions or local tissue trauma. Consequently, any of the following signs or symptoms should be considered with a high degree of suspicion: red/white lesions, ulceration, loose teeth, swelling, pain, or a nonhealing extraction socket. Commonly, masses identified under an ill-fitting denture are attributed to inflammatory conditions (such as an epulis fissuratum), when, in reality, they are SCC. Granular-appearing SCC tissue near a dental endosseous implant can easily be misdiagnosed as peri-implantitis (Figure 1 and Figure 2, page 46). Tumors at more advanced stages will present with a mass or lower lip paresthesia, especially if the inferior alveolar nerve is involved. As a rule, any gingival lesion presenting with the previously described signs and symptoms that has not resolved within 3 weeks to 4 weeks should be biopsied.7–10
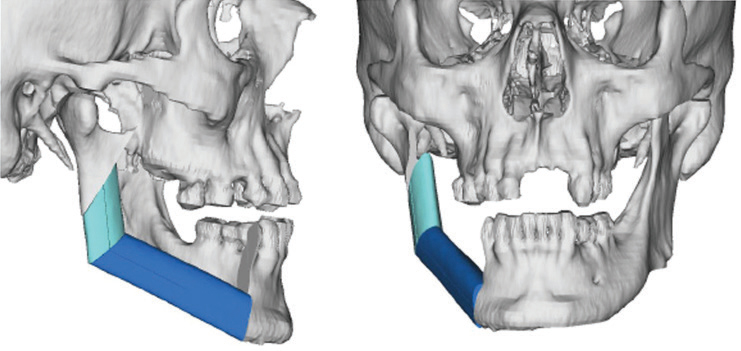
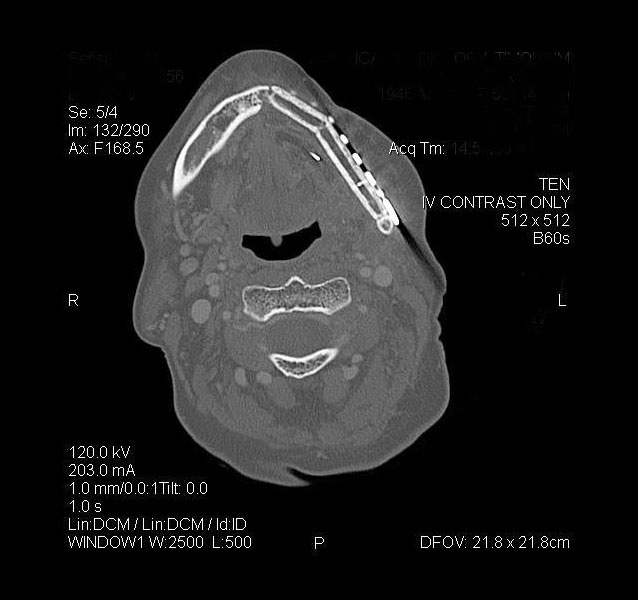
The use of imaging is important to identify bone invasion, as this will affect prognosis. Plain film imaging, such as orthopantomogram and periapical dental films, can be used for initial evaluation, but are considered low-sensitivity tests, as they require at least 30% to 50% bone demineralization to demonstrate bone loss or changes. Computed tomography (CT) and magnetic resonance imaging (MRI) are considered first-line imaging modalities in the preoperative management of gingival SCC.8 These tests can provide important details regarding overlying soft tissue, extent of bone involvement, and regional nodal metastases. A high-resolution, fine-sliced CT scan (1 mm slices) can also be used to provide accurate three-dimensional models for use in virtual surgical planning and advanced reconstructive techniques (Figure 3).11,12 Besides CT, clinicians will also find MRI helpful due to its improved sensitivity and specificity for identifying bone marrow involvement and tumor infiltration of the inferior alveolar nerve. Positron emission tomography is a newer (and more costly) imaging technology that identifies the tumor by its metabolic uptake of a radioactive glucose molecule. This modality is generally useful in the evaluation of regional (cervical node metastases) and distant disease (eg, lung metastases) in advanced stage tumors.8
Initial tumor staging requires a thorough clinical and radiographic examination. Tumors are typically staged using the designated tumor, node, metastasis American Joint Committee on Cancer (AJCC) staging system.13 Difficulty with this classification occurs in relationship to advanced size (T4) and the specific gingival subsite. Alveolar bone invasion can occur early secondary to the thin layer (2 mm to 3 mm) of overlying attached gingiva. Marrow invasion is generally believed to constitute a more aggressive tumor with worse prognosis—however, many clinicians have questioned if tumor infiltration in the alveolar marrow bone meets this definition. Gomez et al1 reported on a series of 83 gingival tumors, and found that bone invasion is an early event and not necessarily an indication of advanced stage disease. Based on numerous studies, the AJCC has modified its staging to report that superficial/alveolar bone invasion does not result in T4a (bone invasion) upstaging because there is inconclusive evidence as to worse prognosis with such alveolar bone involvement.
This debate can be further expanded to patients who present with a positive cancer biopsy in the site of a recent dental extraction. Tumor invasion is generally thought to occur by two methods. In an edentulous individual, the overlying gingiva is involved and invasion occurs through cortical perforations and channels within the bone. In the dentate patient, invasion often occurs via the periodontal ligament and tracks into the basal bone.14 Evaluating bone invasion following a recent extraction can be challenging. It can be difficult to assess if the tooth was mobile secondary to frank bone loss from tumor bone erosion, or if it was secondary to inflammatory periodontitis.8,9,15 Often, the tooth socket has been scaled or treated with bone graft materials, and tumor seeding into the basal bone at the apex of the socket is suspected. Despite this dilemma, prognosis regarding disease recurrence and overall survival has not been established, as there is evidence in the literature in favor of, and against, this hypothesis. As a guide for oral health professionals, if a loose tooth is identified within a gingival mass, it is best not to extract the associated tooth, as this will allow the treating surgeon to assess not only for true bone invasion, but also evaluate the extent of the tumor in the surrounding soft tissue.
MANAGEMENT AND PROGNOSIS
Primary management of gingival SCC involves surgical resection of the associated tumor within the maxilla or mandible.2–4,8 Radiation for this subsite within the oral cavity is usually reserved for the adjuvant setting, based on final pathologic evaluation of the resected specimen. This is especially true in early stage disease, where single modality therapy can be used and avoiding radiation eliminates the risk of osteoradionecrosis (ORN).16–18 Advanced stage disease requires surgery, followed by adjuvant radiotherapy or combined chemoradiotherapy. Prognosis is impacted by the ability to attain a negative surgical margin. Surgical resection involves taking a 1 cm bone and soft tissue margin beyond the visualized tumor and, in the case of a dentate patient, extending the resection to at least one tooth on either side of the tumor. A tumor within the maxillary alveolus requires at least a partial maxillectomy, often with extension into the maxillary sinus or floor of the nose.
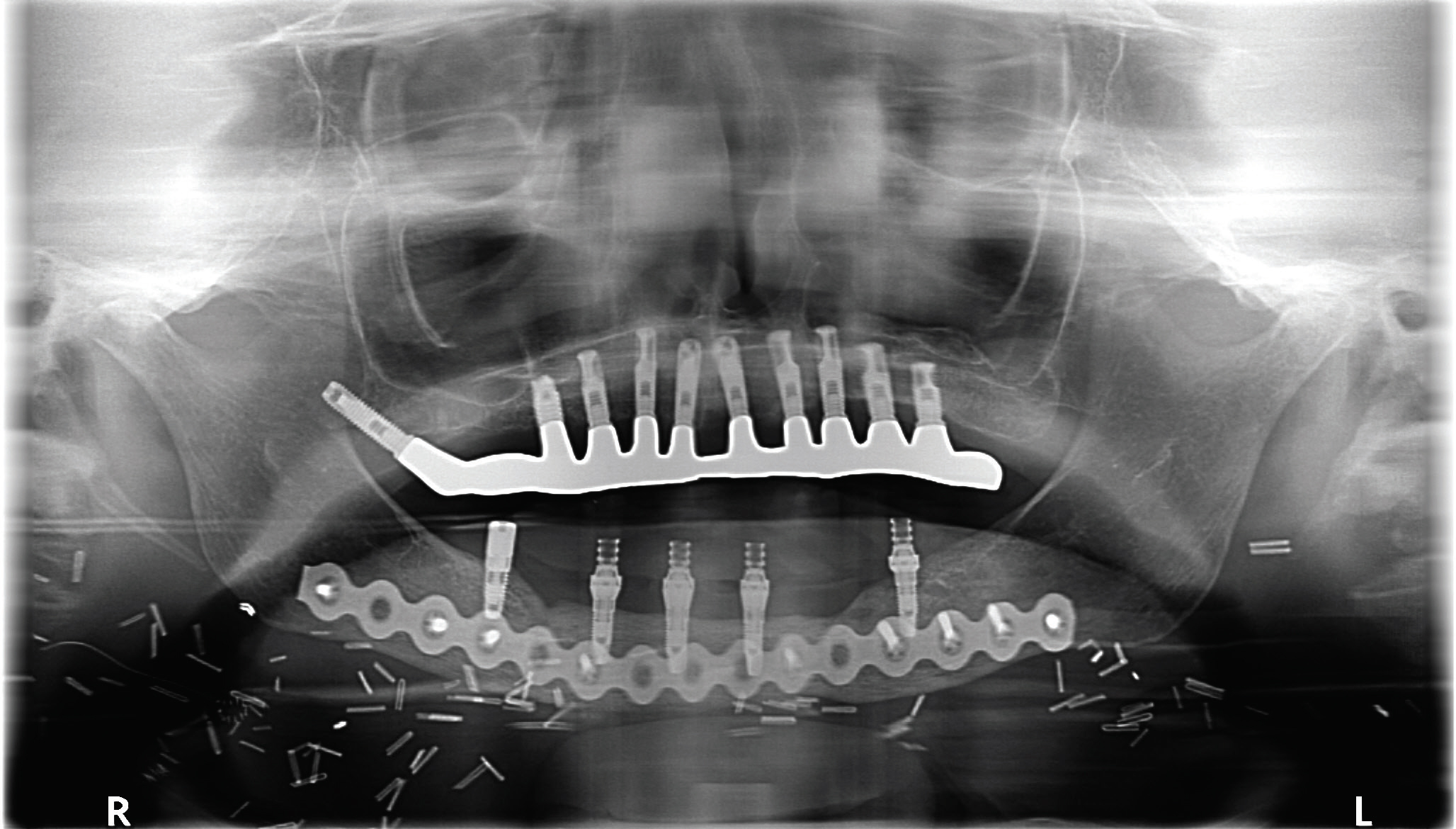
Mandibular resection involves at least removal of the dentoalveolar segment (marginal mandibulectomy) or a composite resection of the entire segment, including the inferior border of mandible (segmental mandibulectomy) in cases involving medullary bone invasion.2–4,8 Other considerations for segmental mandibulectomy include direct invasion of the inferior alveolar nerve, and in cases in which minimal mandibular bone height or thickness will remain, resulting in high risk for post-operative mandibular fracture.19 With smaller defects, reconstruction can be achieved with local flaps and dental prosthesis (denture/obturator), while larger defects will require formal bone and soft tissue vascularized flap reconstruction.8,20,21 This method will provide the framework for facial profile, bone continuity and the possibility of implant-retained dental support (Figure 4 and Figure 5). Free bone grafting becomes more difficult in larger defects (> 4 cm to > 5 cm) or for patients who require adjuvant radiation therapy.8 Dental rehabilitation of these patients requires a team approach involving coordinated care between the reconstructive surgeon and maxillofacial prosthodontist.
In terms of progression-free survival and overall survival, one of the most important negative prognostic factors is the presence of regional cervical nodal metastasis. Historically, with respect to risk of occult nodal metastasis (ie, disease not detected on imaging or clinical exam), SCC of the gingiva was thought to be less aggressive; the decision to perform neck dissection was typically reserved for patients presenting with clinically positive disease or with advanced size cancers (eg, T3 to T4 size). Recent review series have reported the incidence of occult nodal disease to range from 11% to 27% and 10% to 31% within the maxilla and mandible, respectively, with a recommendation from many clinicians to consider elective neck dissection in patients presenting with early stage gingival SCC (T1 to T2).1–4,10,13,15,22
Radiation therapy in the adjuvant setting is typically reserved for advanced stage cancers to improve locoregional control. Newer techniques using intensity-modulated radiotherapy and proton therapy have allowed for more focused techniques, while minimizing the side effects of xerostomia and dysphagia. Independent factors, such as perineural invasion, have been shown to be risk factors for locoregional recurrence and consideration for adjuvant radiotherapy. Two large randomized trials demonstrated the benefit of combined chemoradiotherapy (radiation and cisplatin) for patients with pathologic features that include positive tumor margins, multiple positive lymph nodes, and extranodal extension of disease.23,24 Newer medical therapies involve the use of epidermal growth factor receptor blockers (eg, cetuximab) and immune checkpoint inhibitors (such as nivolumab) for patients who have developed recurrence following failed combined surgery and chemoradiotherapy.25
Surveillance is important to identify recurrent disease, with the hopes of early detection and salvage. Most recurrences of gingival SCC occur within the first 2 years of therapy and surveillance is most aggressive during this time period, with routine evaluations every 2 months. Local recurrences that occur early (within the first 10 months of treatment) can generally be salvaged with surgery, while early regional failures often carry a poorer prognosis—especially in the previously dissected neck.26
ROLE OF ORAL HEALTH PROFESSIONALS
Oral health professionals play an important role during the post-operative phase, not only as a second pair of eyes for tumor surveillance, but also in helping maintain the patient’s oral health. Patients may require preradiation dental restorations or extractions to help prevent ORN. Fluoride trays and routine dental prophylaxis will be required to help prevent radiation caries, as these patients are at high risk secondary to post-radiation xerostomia. The incidence of ORN is reported to be between 5% and 15%, and the role of hyperbaric oxygen therapy has not been established in the prevention or management of this disease. Newer theories as to the cause of ORN have been hypothesized (such as fibroatrophic theory), and the use of tocopherol (vitamin E), and pentoxifylline have become more common in the management of patients requiring dental extraction post-radiation and in the early treatment of ORN.16-18
CONCLUSION
As noted, gingival SCC is an uncommon cancer within the oral cavity that may easily be mistaken for a periodontal disease or local tissue trauma. Many studies regarding treatment are retrospective or based upon the management of other oral cavity SCC subsites. Aggressive surgical therapy that addresses both the management of the primary site and neck is indicated to achieve optimal locoregional control. Oral health professionals can—and should—play a crucial role in the early detection and post-therapy management of this disease.
REFERENCES
- GomezD, Faucher A, Picot V, et al. Outcome of squamous cell carcinoma of the gingiva; a follow-up study of 83 cases. J CraniomaxillofacSurg. 2000;28:331–335.
- ShingakiS, Nomura T,Takada M, Kobayashi T,Suzuki I, Nakajima T.Squamous cell carcinomas of the mandibular alveolus: analysis of prognostic factors. Oncology. 2002;62:17–24.
- BarkR, Mercke C, Munck-WiklandE, Wisniewski N,Hammarstedt-Nordenvall L. Cancer of the gingiva. Eur ArchOtorhinolaryngol. 2016;273:1335– 1345.
- LubekJ, El-Hakim M, Salama AR, LiuX, Ord RA. Gingival carcinoma; retrospective analysis of 72 patients andindications for elective neck dissection. Br J OralMaxillofac Surg. 2011;49:182–185.
- IsayevaT, LiY, MaswahuD, Brandwein-Gensler M. Human papillomavirus in non-oropharyngeal head and neck cancers: a systematic literature review.Head Neck Pathol. 2012;6(Suppl 1):S104–S120.
- HerreroR, Castellsagué X, Pawlita M, et al. Humanpapillomavirus and oral cancer: theInternational Agency for Research onCancer multicenter study. J Natl Cancer Inst. 2003;95:1772– 1783.
- BharanidharanR, Dineshkumar T, Raghavendhar K, Kumar AR. Squamouscell carcinoma of the gingiva: adiagnostic enigma. J Oral MaxillofacPathol. 2015;19:267.
- LubekJE, Magliocca KR. Evaluation of the bonemargin in oral squamous cellcarcinoma. Oral Maxillofac Surg Clin North Am. May 24, 2017. Epub ahead of print.
- YamagataK, Ito H, Onizawa K, Yamatoji M, Yanagawa T,Bukawa H. Prognosis for gingival carcinoma with a delayed diagnosis after dental extraction. J Oral MaxillofacSurg. 2013;71:2189– 2194.
- MuñozGuerra MF, Naval Gías L, CampoFR, Pérez JS. Marginal and segmental mandibulectomy in patientswith oral cancer: a statisticalanalysis of 106 cases. J Oral Maxillofac Surg. 2003;61:1289– 1296.
- MonacoC, Stranix JT,Avraham T, et al. Evolution of surgicaltechniques for mandibular reconstructionusing free fibula flaps: the next generation. Head Neck. 2016;38(Suppl 1):E2066– E2073.
- RodbyKA, Turin S, Jacobs RJ, et al. Advances inoncologic head and neck reconstruction: systematic review and future considerations of virtual surgical planning and computeraided design/computer aided modeling. J Plast ReconstrAesthet Surg. 2014;67:1171–1185.
- Amin MB, Edge S, Greene F, et al, eds. AJCC CancerStaging Manual. 8th ed. New York, NY: Springer; 2017.
- McGregorAD, MacDonald DG. Routes ofentry of squamous cell carcinoma tothe mandible. Head Neck Surg. 1988;10:294–301.
- OverholtSM, Eicher SA, Wolf P, Weber RS. Prognosticfactors affecting outcome in lower gingival carcinoma. Laryngoscope. 1996;106:1335–1339.
- AnnaneD, Depondt J, Aubert P, et al. Hyperbaric oxygen therapy for radionecrosis ofthe jaw: a randomized, placebo-controlled double-blind trial from the ORN96 study group. J Clin Oncol. 2004;22:4893–4900.
- DelanianS, Chatel C, Porcher R, Depondt J, LefaixJL. Complete restoration of refractory mandibular osteonecrosis by prolonged treatment with a pentoxifylline tocopherol clodronate combination (PENTOCLO): aphase II trial. Int J Radiat Oncol Biol Phys. 2011;180:832–839.
- LubekJE, Hancock MK, Strome SE. What is the value of hyperbaric oxygen therapy inmanagement of osteoradionecrosis of the head and neck?Laryngoscope. 2013;123:555–556.
- BarttelbortSW, Bahn SL, Ariyan S. Rim mandibulectomy for cancer of the oral cavity.Am J Surg. 1987;154:423–428.
- ChigurapatiR, Aloor N, Salas R, Schmidt BL. Quality of lifeafter maxillectomy and prosthetic obturator rehabilitation. J Oral MaxillofacSurg. 2013;71:1471–1478.
- BrownJS, Rogers SN,McNally DN, Boyle M. A modifiedclassification for the maxillectomy defect. Head Neck. 2000;22:17–26.
- MontesDM, Carlson ER, Fernandes R, et al. Oralmaxillary squamous carcinoma: an indicationfor neck dissection in the clinicallynegative neck. Head Neck. 2011;33:1581–1585.
- BernierJ, Domenge C, Ozsahin M, et al. Postoperativeirradiation with or without concomitant chemotherapy for locallyadvanced head and neck cancer. N Engl J Med. 2004;350:1945–1952.
- CooperJS, Pajak TF,Forastiere AA, et al. Postoperativeconcurrent radiotherapy andchemotherapy for high-risk squamous cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–1944.
- FerrisRL, Blumenschein G Jr., Fayette J, et al.Nivolumab for recurrent squamous cell carcinoma of the head andneck. N Engl J Med. 2016;375:1856–1867.
- LiaoCT, Chang JT, Wang HM, et al. Salvage therapy in relapsed squamous cell carcinoma of the oral cavity: how and when?Cancer. 2008;112:94–103.
Featured photo by CIPHOTOS/ISTOCK/GETTY IMAGES PLUS
From Dimensions of Dental Hygiene. December 2017;15(12):45-48.



