Dentinal Hypersensitivity Management
Dental hygienists can help their patients effectively address this common source of discomfort by staying abreast of the latest treatment strategies.
Dentinal hypersensitivity is a painful condition that affects up to 57% of the adult population in the United States and is one of the most frequent complaints of those seeking dental treatment.1 Women age 20 to 40 with meticulous oral hygiene are at the greatest risk of developing sensitivity, which most commonly occurs in the canines and first premolars.2 According to the widely recognized hydrodynamic theory, the pain sensation associated with dentinal hypersensitivity is initiated by changes in the volume or flow of fluid in dentin tubules that trigger impulses to the nerve and/or pulp.3,4
SIGNS AND SYMPTOMS
Many patients receive preventive dental hygiene care twice per year or periodontal maintenance four times or more per year, depending on their needs. Dental hygienists are well positioned to effectively monitor sensitivity, which is done through the use of air, tactile, and thermal stimuli during routine procedures. Dental hygienists need to stay adequately informed in identifying sensitivity, assisting with its diagnosis, and helping to manage the symptoms with in-office treatments and/or self-care therapies.
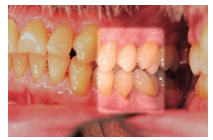
Symptoms of dentinal hypersensitivity may manifest sporadically. Certain types of abrasive dentifrices can remove mineral content of enamel, leaving dentin tubules exposed.5 In addition to enamel wear by attrition, abrasion (Figure 1), and erosion, sensitivity is also related to predisposing factors, such as the presence of periodontal diseases and related treatment, inadequate alveolar bone, or thin biotype.6,7 Erosion is considered the primary cause of sensitivity and can be extrinsic or intrinsic in origin. Extrinsic factors include acidic foods, chemical exposure, improper tooth whitening procedures, or the use of mouthrinses with low pH. Gastric fluids, chronic alcohol use, and eating disorders are examples of intrinsic contributors.1
Due to difficulties in distinguishing dentinal hypersensitivity from other conditions, it is important to utilize a variety of diagnostic and evaluation techniques to assess possible causes of dental/pulpal pain.8 Certain pathological conditions can also cause pain that is not associated with sensitivity. Clinicians should rule out the pain caused by caries, cracked tooth syndrome, restoration leakage, traumatic occlusion, or post-operative sensitivity. Additionally, pulpitis, gingival inflammation, and fractured restorations should be considered when diagnosing dentinal hypersensitivity.1,5,6 Radiographic and periodontal assessments, pulp vitality testing, and evaluation of occlusion will provide important information that can help clinicians rule out alternative causes for the sensitivity.
If dentinal hypersensitivity is caused by erosion or abrasion, the initial approach should include the taking of a patient’s medical history and screening in order to identify any predisposing or etiologic contributors. This should be followed by dietary and oral hygiene counseling to manage abrasion.5 Oral health professionals should also consider gathering the following information from the patient: frequency and length of brushing, type of dentifrice used, intervals of brush replacement, and estimated force used (Table 1).1,2
MANAGEMENT APPROACHES
At this time, a number of product choices are available to reduce or eliminate dentinal hypersensitivity, and they can be categorized by two mechanisms of action. The first is nerve depolarization through the use of nitrates, and the second involves occlusion of exposed dentinal tubules through plug formation (Figure 2 and Figure 3). Strontium compounds, fluoride, calcium phosphate technologies, potassium oxalate, resin sealers, glutaraldehyde, and lasers have been used to occlude open dentinal tubules.5 Regarding the efficacy of oxalate-containing products, a recent study demonstrated a decrease in sensitivity when a mouthrinse containing 1.4% potassium oxalate was utilized when compared to a fluoride dentifrice used alone.9 Glutaraldehyde has also been shown to provide relief of pain caused by dentinal hypersensitivity.10

Potassium nitrate is the primary active agent for depolarizing stimulated nerves to interrupt the pain sensation.6 Potassium nitrate is effective, however, it takes approximately 4 weeks to 8 weeks to provide desensitizing efficacy.5 Patients need to be informed about this fact, and encouraged to continue using potassium nitrate dentifrices as directed in order to achieve the desired results.
If patients require therapy that will provide immediate relief, the in-office application of an arginine prophylaxis paste may be the most effective strategy. Arginine is an amino acid that may provide both immediate and lasting relief of dentinal hypersensitivity.6,11 Research shows that when calcium carbonate is combined with arginine, remineralization occurs and dentin tubules are occluded at a depth of 2 mm.5
Many patients experience post-treatment sensitivity following periodontal procedures. The dentinal hypersensitivity can be identified and assessed using tactile, air-blast, and thermal stimuli. A clinical study reported that the application of an 8% arginine prophylaxis paste with calcium carbonate was effective immediately, as well as 1 month after application. In all three stimuli tests, the arginine group exhibited a statistically significant reduction in sensitivity.12 Another study showed that a single application of a prophylaxis paste containing 8% arginine with calcium carbonate achieved a 71.7% reduction in sensitivity with air-blast testing and 84.2% reduction with tactile stimulus testing.5 In studies comparing both pre- and post-procedural application, hypersensitivity decreased significantly. The arginine prophylaxis paste was shown to be more effective when compared to fluoride-free and calcium carbonate prophylaxis paste alone during a single, professional application.13–15

Arginine-containing dentifrice also has been shown to be effective in managing sensitivity.16,17 This product, however, is not currently available in the US. A mouthrinse containing 0.8% arginine and 0.05% sodium fluoride is currently on the market, and a statistically significant reduction in dentinal hypersensitivity was observed after 8 weeks of use.18
In vitro studies report that hydroxyapatite helped reduce sensitivity when added to an ordinary dentifrice as it replicated the protective features of the oral environment.19–21 Dentifrices with and without hydroxyapatite were applied to dentin discs. The dentifrice containing hydroxyapatite was significantly more effective in tubule occlusion than the ordinary dentifrice. The tubule-occluding rate was greater than 90% in the group of dentifrice containing hydroxyapatite.19
A 3-day clinical study investigated the desensitizing properties of three dentifrices: 8% arginine with 1,450 ppm sodium fluoride sodium monofluorophosphate; 8% strontium acetate with 1,040 ppm sodium fluoride; and 30% micro-aggregation of zinc-carbonate hydroxyapatite nanocrystals. All three dentifrices demonstrated improvement in air-blast, cold water, tactile, and subjective test scores, suggesting that the hydroxyapatite product shows promise for the management of sensitivity.20 During active whitening treatment using 7% hydrogen peroxide gel, Browning et al21 found a reduction in the number of days that patients experienced sensitivity when nanohydroxyapatite paste was used for 5 minutes immediately following whitening treatment.
Calcium phosphate technologies are another option in the treatment of dentinal hypersensitivity. Amorphous calcium phosphate (ACP) is an ingredient available in both a gel and in bleaching products that makes calcium and phosphate ions available in saliva, which may encourage remineralization.22 Casein phosphopeptide-ACP, or Recaldent®, is a milk protein derivative that can be formed in the mouth and mimics the natural remineralization process. One study evaluated the effects of Recaldent paste on sensitivity and found that it was ineffective when used short-term;23 however, results of another study showed that topical placement of Recaldent reduced dentinal hypersensitivity through the deposition of calcium phosphate compounds onto the tooth structure.19
Calcium sodium phosphosilicate or NovaMin® was originally developed for bone regeneration and has been used to occlude open dentin tubules, thus relieving sensitivity pain. It is compatible with the human body and reacts with oral moisture to imitate the mineralization on the exposed dentin.24 One study looked at dentifrices containing NovaMin, 5% potassium nitrate, and 0.4% stannous fluoride. Measurements of dentinal hypersensitivity were recorded at 2 weeks, 4 weeks, and 12 weeks. All three products were considered effective in reducing sensitivity; however, the NovaMin toothpaste demonstrated a more significant effect after 2 weeks and 4 weeks.25 An in vitro study showed tubule occlusion with 5% NovaMin.26 A recent in vitro scanning electron microscopy (SEM) study compared tubule occlusion after a single application of NovaMin and glutaraldehyde. Both were effective but NovaMin demonstrated more completely occluded tubules.27
The most recent addition to the calcium phosphate arena is tri-calcium phosphate, which slowly releases calcium to the tooth surface. It is designed to improve the remineralizing effects of fluoride, while also decreasing dentinal hypersensitivity.28
New options are available to treat dentinal hypersensitivity in the dental office, including a varnish containing 5% glutaraldehyde and 35% hydroxyethylmethacrylate and surface pre-reacted glass-ionomer fillers. At this time, more research is needed to determine their efficacy in treating hypersensitivity.
LASER USE FOR SENSITIVITY MANAGEMENT
According to the American Dental Association, dental practitioners are allowed to use lasers within their scope of practice and once appropriate training/certification has been obtained.29 In some states, dental hygienists can use lasers as an adjunct to periodontal therapy to reduce bacteria levels in pockets and facilitate hemostasis. Dental hygienists should check with their individual state boards before treating any patient with lasers to ensure they are adhering to their scope of practice.30
Current research on the use of lasers to reduce dentinal hypersensitivity is limited;6 however, a few studies have evaluated the effectiveness of Er:YAG, Nd:YAG, and diode lasers. In an evaluation of the occluding effect of a laser and dentifrice, four groups were compared.31 Group one received no treatment; group two used a toothpaste containing 20% nano-carbonate apatite (n-CAP); group three received treatment with an Er:YAG laser; and group four underwent laser treatment following the application of the n-CAP toothpaste. SEM confirmed that dentin tubule occlusion was highest (87%) with n-CAP alone. The study concluded that the combination method has potential for improved tubule occlusion, but further research is needed.31
A clinical study found that the combination of a Nd:YAG laser and glutaraldehyde desensitizer was successful in treating sensitivity both in the short- and long-term.32 Raichur et al33 determined that the diode laser was more effective than stannous fluoride and potassium nitrate gels at relieving dentinal hypersensitivity. The combination of a diode laser and 2% sodium fluoride solution showed a significant reduction in discomfort when baseline values were compared to all post-treatment evaluations.34
A systematic review of laser use as a desensitizing procedure showed no evidence of detrimental effects in five of the eight studies that assessed clinical complications, allergic reactions, or effects on the pulp. The reviewers did, however, conclude that conflicting reports exist.35
CONCLUSION
Dental hygienists play an important role in the diagnosis and management of dentinal hypersensitivity through preventive counseling, recommendation of self-treatment products, and application of in-office agents. Dental hygienists can offer behavior modification strategies with integration of appropriate products to cope with symptoms of sensitivity. With knowledge of the etiology of dentinal hypersensitivity and an adequate understanding of available management options, dental hygienists will be able to help provide patient-centered, evidence-based care.
REFERENCES
- Trushkowsky RD, Garcia-Godoy F. Dentin hypersensitivity: differential diagnosis, tests, and etiology. Compend Contin Educ Dent. 2014;35:99–104.
- Daniel SJ, Harfst SA, Wilder RS. Dental Hygiene Concepts, Cases, and Competencies. 2nd ed. St. Louis: Mosby Elsevier; 2008:623–637.
- Li Y. Dentin hypersensitivity: diagnosis and strategic approaches. Available at: cdeworld.com/courses/4674. Accessed March 18, 2015.
- Brannstrom M. The elicitation of pain in human dentine and pulp by chemical stimuli. Arch Oral Biol. 1962;7:59–62.
- Wolff M. Science behind the sensitivity: science-supported therapy for the effective relief of dentin hypersensitivity. Journal of Clinical Dentistry. 2009;20(Special Issue):23–31.
- Li Y. Innovations for combating dentin hypersensitivity: current state of the art. Compend Contin Educ Dent. 2012;33(Spec No 2):10–16.
- West NX, Lussi A, Seong J, Hellwig E. Dentin hypersensitivity: pain mechanisms and aetiology of exposed cervical dentin. Clin Oral Investig. 2013;17(Suppl 1):S9–19.
- Gernhardt C. How valid and applicable are current diagnostic criteria and assessment methods for dentin hypersensitivity? An overview. Clin Oral Investig. 2013;17(Suppl 1):S31–S40.
- Sharma D, McGuire JA, Amini P. Randomized trial of the clinical efficacy of a potassium oxalate-containing mouthrinse in rapid relief of dentin sensitivity. J Clin Dent. 2013;24:62–67.
- Vora J, Mehta D, Meena N, Sushma G, Finger WJ, Kanehira M. Effects of two topical desensitizing agents and placebo on dentin hypersensitivity. Am J Dent. 2012;25:293–298.
- Cummins D. Recent advances in dentin hypersensitivity: clinically proven treatments for instant and lasting sensitivity relief. Am J Dent. 2010;23(Spec No A):3A–13A.
- Uraz A, Erol-Simsek O, Pehlivan S, Suludere Z, Bal B. The efficacy of 8% arginine-CaCO3 applications on dentine hypersensitivity following periodontal therapy: a clinical and scanning electron microscope study. Med Oral Patol Oral Cir Bucal. 2013;18:298–305.
- Collins JR, Richardson D, Sotero K, Mateo LR, Mauriz I. Beneficial effects of an arginine-calcium carbonate desensitizing paste for treatment of dentin hypersensitivity. Am J Dent. 2013;26:63–67.
- Kapferer I, Pflug C, Kisielewsky I, Giesinger J, Beier US, Dumfahrt H. Instant dentin hypersensitivity relief of a single topical application of an in-office desensitizing paste containing 8% arginine and calcium carbonate: a split-mouth, randomized-controlled study. Acta Odontol Scand. 2013;71:994–999.
- Tsai WS, Placa SJ, Panagoakos FS. Clinical evaluation of an in-office desensitizing paste containing 8% arginine and calcium carbonate for relief of dentin hypersensitivity prior to dental prophylaxis. Am J Dent. 2012;25:165–170.
- Li Y, Lee S, Zhang YP, Delgado E, DeVizio W, Mateo LR. Comparison of clinical efficacy of three toothpastes in reducing dentin hypersensitivity. J Clin Dent. 2011;22:113–120.
- Yan B, Yi J, Li Y, Chen Y, Shi Z. Arginine-containing toothpastes for dentin hypersensitivity: systematic review and meta-analysis. Quintessence Int. 2013;44:709–723.
- Hu D, Stewart B, Mello S, et al. Efficacy of a mouthwash containing 0.8% arginine, PVM/MA copolymer, pyrophosphates, and 0.05% sodium fluoride compared to a negative control mouthwash on dentin hypersensitivity reduction. A randomized clinical trial. J Dent. 2013;41(Suppl 1):S26–S33.
- Yuan P, Shen X, Liu J, et al. Effects of dentifrice containing hydroxyapatite on dentinal tubule occlusion and aqueous hexavalent chromium cations sorption: a preliminary study. PLoS One. 2012;7:45283.
- Orsini G, Procaccini M, Manzoli L, et al. A 3-day randomized clinical trial to investigate the desensitizing properties of three dentifrices. J Periodontol. 2013;84:65–73.
- Browning WD, Cho SD, Deschepper EJ. Effect of nano-hydroxyapatite paste on bleaching-related tooth sensitivity. J Esthet Restor Dent. 2012;24:268–276.
- Orchardson R, Gillam DG. Managing dentin hypersensitivity. J Dent Assoc. 2006;137:990–998.
- Kowalczyk A, Botulinski B, Jaworska M, Kierklo A, Pawinska M, Dabrowska E. Evaluation of the product based on Recaldent technology in the treatment of dentin hypersensitivity. Adv Med Sci. 2006;51(Suppl 1):40–42.
- Litkowski L, Greenspan DC. A clinical study of the effect of calcium sodium phosphosilicate on dentin hypersensitivity—proof of principle. J Clin Dent. 2010;21:77–81.
- Sharma N, Roy S, Kakar A, Greenspan DC, Scott R. A clinical study comparing oral formulations containing 7.5% calcium sodium phosphosilicate (NovaMin), 5% potassium nitrate, and 0.4% stannous fluoride for the management of dentin hypersensitivity. J Clin Dent. 2010;21:88–92.
- Salian S, Thakur S, Kulkarnai S, LaTorre G. A randomized controlled clinical study evaluating the efficacy of two desensitizing dentifrices. J Clin Dent. 2010;21:82–87.
- Joshi S, Gowda AS, Joshi C. Comparative evaluation of NovaMin desensitizer and Gluma desensitizer on dentinal tubule occlusion: a scanning electron microscopic study. J Periodontal Implant Sci. 2013;43:269–275.
- Karlinsey RL, Mackey AC. Solid-state preparation and dental application of an organically-modified calcium phosphate. J Mater Sci. 2009;44:346–349.
- American Dental Association Council on Scientific Affairs. Statement on Lasers in Dentistry. Available at: ada.org/en/about-the-ada/ada-positions-policies-and-statements/statement-on-lasers-in-dentistry. Accessed March 18, 2015.
- Pope JD, Rossman JA, Treating periodontal diseases with laser therapy. Dimensions of Dental Hygiene. 2014;12(6):53–59.
- Han SY, Jung HI, Kwon HK, Kim BI. Combined effects of Er:YAG and nano-carbonate apatite dentifrice on dentinal tubule occlusion: in vitro study. Photomed Laser Surg. 2013;31:342–348.
- Lopes AO, Arahha AC. Comparative evaluation of the effects of Nd:YAG laser and a desensitizer agent on the treatment of dentin hypersensitivity: a clinical study. Photomed Laser Surg. 2013;31:132–138.
- Raichur PS, Setty SB, Thakur SL. Comparative evaluation of diode laser, stannous fluoride gel, and potassium nitrate gel in the treatment of dentinal hypersensitivity. Gen Dent. 2013;61:66–71.
- Femiano F, Femiano R, Lanza A, Festa MV, Rullo R, Perillo L. Efficacy of diode laser in association to sodium fluoride vs Gluma desensitizer on treatment of cervical dentin hypersensitivity: a double blind controlled trial. Am J Dent. 2013;26:214–218.
- Blatz MB. Laser therapy may be better than topical desensitizing agents for treating dentin hypersensitivity. J Evid Based Dent Pract. 2012;12(Suppl 3): 220–230.
From Dimensions of Dental Hygiene. April 2015;13(4):25–26,28,30,32.

