
Components of Optimal Ultrasonic Therapy
The use of proper technique during ultrasonic therapy may help prevent over instrumentation and encourage positive outcomes.
In today’s sophisticated practice settings, ultrasonic instrumentation is routinely incorporated unless contraindications prevail. Its use in oral prophylaxis, nonsurgical periodontal therapy, and periodontal maintenance provides many potential advantages (Table 1). Like hand instrumentation, however, the risk of overinstrumentation is possible with ultrasonic technology.1–10
Overinstrumentation is a clinician-created or iatrogenic problem stemming from the use of improper technique or debriding more than is necessary to encourage healing of adjacent tissues. Over instrumentation can include creating root defects, leaving roughness, and removing unnecessary tooth structure—each of which may encourage biofilm accumulation and tooth sensitivity.11 A smooth root or implant surface reduces the likelihood that plaque biofilms will reattach and, thus, may decrease the risk of periodontal diseases, peri-mucositis, and peri-implantitis.

The goal of debridement is to remove plaque biofilms, their byproducts, their retentive factors, and calculus-embedded cementum to create a biologically acceptable root surface conducive to optimal healing, while preventing unnecessary loss of tooth surface. As such, the point of overinstrumentation may be different for each patient. During nonsurgical periodontal therapy, the patient’s response to individualized therapy is evaluated throughout multiple appointments and at the reevaluation visit. At these times, additional instrumentation may be indicated due to tactile interpretation, bleeding points, visible inflammation, or endoscopic observation of calculus. This therapy is justified and is not considered overinstrumentation.
RELEVANT RESEARCH
Researchers continue to investigate the causes of root defects, roughness, and unnecessary tooth surface removal with mechanical root debridement. In regard to root defects, power setting, load of the ultrasonic insert/tip (UIT) on the tooth, and the cross-sectional shape of UITs may affect dentin defects with both magnetostrictive and piezoelectric ultrasonic technology.12 Casarin et al1 found that hand and ultrasonic instrumentation produced the same level of defect depths, regardless of power settings. As both power and manual instrumentation create alterations in the dentin surfaces under the best of conditions,1 optimal outcomes are achieved by using proper instrumentation technique.
The amount of root roughness remaining after instrumentation has also been examined. Singh et al8 concluded that manual and ultrasonic instrumentation methods were equal in debridement efficacy, while other studies revealed that ultrasonic instrumentation left a smoother surface than curets.3,5,7 Marda et al5 found that magnetostrictive inserts created reduced root surface roughness, less root surface removal, and better efficiency of calculus removal than a rotary bur or curets.
Yousefimanesh et al10 concluded that the exertion of different lateral forces in both magnetostrictive and piezoelectric devices caused similar effects on tooth surfaces. In this in vitro study, root surface roughness was the same after both types of ultrasonic technology were used, though the piezoelectric device produced the least roughness when 200 g of pressure were applied.10 When piezoelectric, magnetostrictive, and curet instrumentation was compared, each removed approximately the same amount of calculus.7 Instrumentation with curets, however, produced the roughest surface.7 Kawashima et al3 reported that ultrasonic instrumentation methods produced a smoother surface than the curet, and that there was a difference in root surface roughness after two different piezoelectric tips were used.
Results of such studies are not directly comparable, however, due to the various methodologies employed. Clinicians are encouraged, nevertheless, to heed recommendations regarding prevention of overinstrumentation due to its negative effects on clinical outcomes.
Additional research has been performed on the effect of coated UITs designed for root surfaces and implants. Vastardis et al13 found that diamond-coated UITs left a rougher root surface and removed more root surface compared to hand instruments and a nondiamond-coated UIT, with the nondiamond-coated UIT producing the smoothest root surface. In a study by Ribeiro et al,14 results showed that a sonic diamond-coated tip and a standard UIT produced more roughness than a curet. The use of diamond-coated UITs without an endoscope or outside of surgical intervention is not recommended because of the likelihood for overinstrumentation. Additional studies evaluating which coatings produce the least amount of roughness after instrumentation are ongoing.
In regard to root surface removal, Mishra and Prakash6 compared laser therapy to ultrasonic and hand instrumentation using extracted teeth. Ultrasonic instrumentation yielded surfaces devoid of deposits and root surfaces that were essentially unchanged.6 Hand instrumentation removed more root surface than other methods, however, the surfaces were smooth.6 The laser provided similar outcomes in calculus removal as both ultrasonic and hand instrumentation, but it also caused greater loss of tooth surface and left more surface roughness than the other techniques. Santos et al7 demonstrated that curets removed more root surface tissue than an ultrasonic device. These data support the benefits of ultrasonic devices in removing the least amount of root surface while achieving the desired clinical outcome.
Dental hygienists must understand the five aspects of quality ultrasonic therapy in order to prevent overinstrumentation. These factors include: power setting, tip angulation, lateral pressure, exposure time, and tip wear (Table 2). The interaction of one or more of these factors results in the increased probability of overinstrumentation.
POWER SETTING

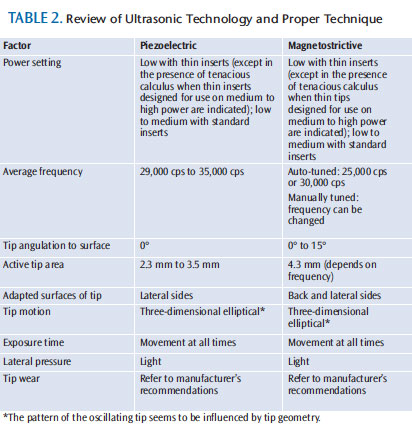
Augmenting the power setting increases the distance the active tip travels across the tooth from top to bottom of the stroke (Figure 1)—though not necessarily in a linear fashion.4 This movement creates a three-dimensional “chipping” of the tip against the surface, often referred to as displacement amplitude. High-power settings and long strokes remove more calculus,4 but also increase the probability of patient discomfort and overinstrumentation.
Low-power settings may create a better relationship between deposit removal, minimal loss of dentin, and surface roughness.4 The UIT should be tuned and the power setting increased as needed, depending on the deposit’s tenacity. The use of low power with thin tips, however, can easily burnish calculus to a smooth veneer, making it almost impossible to remove. Therefore, thin UITs should not be utilized to remove heavy or tenacious calculus unless they are intended for use at high-power levels and have the ability to break deposits cleanly from the root surface.15 When high-power settings are implemented, adequate pain control is essential to successful instrumentation.15
Power (length of stroke) and frequency (speed) are distinct aspects of ultrasonic instrumentation that work together when the energy is activated on a tooth. Frequency is how fast the tip moves across the surface in cycles-per-second, and it can be adjusted only with a manual-tuned unit. This adjustment allows the practitioner to balance the power and frequency for maximum effectiveness and comfort.
TIP ANGULATION
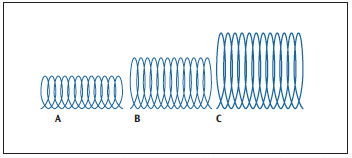
Incorrect application of the tip during instrumentation may cause undesirable surface alterations. Tip angulation must be mentally visualized because of the inability to use direct vision with subgingival debridement without an endoscope, and due to tip size obstructing the view of angulation. The greater the angle between the active tip and the root surface, the greater the energy output and chance of overinstrumentation.1 The active tip to tooth angle should be maintained at 15° or less (Figure 2). To prevent severe root damage, piezoelectric units should be used at close to 0° angulation.11,16
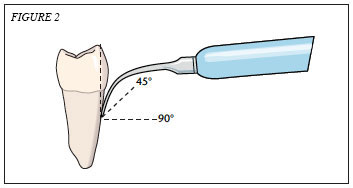
In addition to tip angulation, adapting the terminal 2 mm to 4 mm is essential because this is the active tip area (Figure 3). The precise recommendation for active tip adaptation depends on the type of technology and the unit (refer to the manufacturer’s instructions for information on the active tip area).
Correct adaptation of the tip is also crucial. With magnetostrictive inserts, although all sides of the tip are active, the lateral surfaces or back should be adapted because they generate the least amount of energy, which helps prevent overinstrumentation. Adapting the point of an insert to a root surface devoid of calculus may cause striations in the root structure or overinstrumentation.
The lateral sides of piezoelectric tips are the most effective. Though each mechanism requires adaptation of the lateral sides, the manner of movement—both loaded and unloaded—results in an elliptical motion. Oscillation patterns are not dependent on whether the unit uses piezoelectricity or magnetostriction; instead, power setting and shape/design of the UIT influence the patterns.17,18
LATERAL PRESSURE
Calculus removal is less effective when strong lateral pressure is used. Strong lateral pressure interferes with the free movement of the tip to fracture deposits, and it can diminish the activation. Only light lateral pressure is needed; just enough to balance the instrument and let it work independently. Grasp pressure may also be related to root roughness/defects.11 Heavy pressure causes the tip to create roughness and remove more structure than necessary. An extraoral grasp with thin inserts is recommended, which helps maintain a light hold on the instrument and balance on the fulcrum. The majority of patients who do not begin therapy with sensitivity should not develop it post-treatment when a light touch is used.
EXPOSURE TIME
The longer the UIT’s exposure time on a specific area, the greater the risk of negative effects. To avoid this pitfall, clinicians must keep the UIT?moving at all times. The moving strokes should overlap and be multidirectional. Patients who are not indicated for pain control should not feel sensitivity while the UIT?is moved constantly over the surface if tuning and instrumentation technique are correct, and the likelihood of iatrogenic damage should also be reduced.
TIP WEAR
Tip wear may affect the performance of UITs by reducing their displacement amplitude.19 With a magnetostrictive insert, wear is evaluated by examining the stack and working ends for damage. With both piezoelectric and magnetostrictive instruments, the length of the working end is also assessed. Shortening of the working end reduces efficiency, lengthens instrumentation time, creates the need to use increased power settings, and raises the risk for tip fracture. Unfortunately, a 1 mm loss of the working end length results in 25% less efficiency. A 2 mm loss results in 50% less efficiency, at which point tip replacement is indicated.19, Efficiency guides are available from manufacturers to objectively assess tip wear.
Clinicians should use UITs that work at optimal levels and have not outlived their usefulness. A recent study found that significantly more root surface roughness was found when worn UITs were implemented vs new UITs, and the roughness change was dependent on angle of application and power setting.20 Arabaci et al20 concluded that tip wear is just as important as other factors in preventing surface roughness.
CONCLUSION
A review of the literature assessing the use of power-driven and hand-delivered instrumentation reveals similar clinical outcomes.21 Just as with hand instrumentation, clinicians may overtreat roots with ultrasonic therapy. In addition to causing iatrogenic problems with the tooth, overinstrumentation can lead to discomfort and sensitivity, as well as loss of efficiency20 during care. The manufacturer instructions for proper use of the unit should always be followed. Patients’ nonverbal and verbal cues, such as grimaces, may indicate pain.
In the future, UITs with different materials might be recommended depending on the status of the root surface. For example, Rühling et al22 found that a teflon-coated sonic tip removed less root surface than a curet or traditional UITs, and might be an alternative for periodontal maintenance patients. In the study, a curet and a conventional sonic scaler insert removed the most root surface compared to a piezoelectric tip.22 As use of the dental endoscope grows in conventional therapy, clinicians will be able to assess the factors of optimal instrumentation while analyzing the deposit removal effectiveness, and more in vivo research will be conducted.
Analysis of the risk of overinstrumentation with ultrasonic therapy is difficult but necessary. The occurrence of overinstrumentation can be prevented using the best practices recommended by researchers, manufacturers, and clinicians. By adhering to the recommendations regarding the key factors of quality ultrasonic instrumentation, clinicians can provide both effective and safe care to their patients.
REFERENCES
- Casarin RC, Ribeiro FV, Sallum AW, Sallum EA, Nociti-Jr FH, Casati MZ. Root surface defect produced by hand instruments and ultrasonic scaler with different power settings: an in vitro study. Braz Dent J. 2009;20:58–63.
- Dahiya P, Kamal R, Gupta R, Pandit N. Comparative evaluation of hand and power-driven instruments on root surface characteristics: A scanning electron microscopy study. Contemp Clin Dent. 2011;2:79–83.
- Kawashima H, Sato S, Kishida M, Ito K. A comparison of root surface instrumentation using two piezoelectric ultrasonic scalers and a hand scaler in vivo. J Periodontal Res. 2007;42:90–95.
- Lampe Bless K, Sener B, Dual J, Attin T, Schmidlin PR. Cleaning ability and induced dentin loss of a magnetostrictive ultrasonic instrument at different power settings. Clin Oral Investig. 2011;15:241–248.
- Marda P, Prakash S, Devaraj CG, Vastardis S. A comparison of root surface instrumentation using manual, ultrasonic and rotary instruments: an in vitro study using scanning electron microscopy. Indian J Dent Res. 2012;23:164–170.
- Mishra MK, Prakash S. A comparative scanning electron microscopy study between hand instrument, ultrasonic scaling and erbium doped:Yttrium aluminum garnet laser on root surface: A morphological and thermal analysis. Contemp Clin Dent. 2013;4:198–205.
- Santos FA, Pochapski MT, Leal PC, Gimenes-Sakima PP, Marcantonio E Jr. Comparative study on the effect of ultrasonic instruments on the root surface in vivo. Clin Oral Investig. 2008;12:143–150.
- Singh S, Uppoor A, Navak D. A comparative evaluation of the efficacy of manual, magnetostrictive and piezoelectric ultrasonic
- Solís Morenoinstruments—an in vitro profilometric and SEM study. J Appl Oral Sci. 2012;20:21–26.C, Santos A, Nart J, Levi P, Velásquez A, Sanz Moliner J. Evaluation of root surface microtopography following the use of four instrumentation systems by confocal microscopy and scanning electron microscopy: an in vitro study. J Periodontal Res. 2012;47:608–615.
- Yousefimanesh H, Robati M, Kadkhodazadeh M, Molla R. A comparison of magnetostrictive and piezoelectric ultrasonic scaling devices: an in vitro study. J Periodontal Implant Sci. 2012;42:243–247.
- Arabaci T, Cicek Y, Canakci C. Sonic and ultrasonic scalers in periodontal treatment: a review. Int J Dent Hyg. 2007;5:2–12.
- Lea SC, Felver B, Landini G, Walmsley AD. Ultrasonic scaler oscillations and tooth-surface defects. J Dent Res. 2009;88:229–234.
- Vastardis S, Yukna RA, Rice DA, Mercante D. Root surface removal and resultant surface texture with diamond-coated ultrasonic inserts: an in vitro and SEM study. J Clin Periodontol. 2005;32:467–473.
- Ribeiro FV, Casarin RC, Nociti Júnior FH, Sallum EA, Sallum AW, Casati MZ. Comparative in vitro study of root roughness after instrumentation with ultrasonic and diamond tip sonic scaler. J Appl Oral Sci. 2006;14:124–129.
- Pattison AM. Keys to effective calculus removal. Dimensions of Dental Hygiene. 2011;9(10):50–53.
- Flemmig TF, Petersilka GJ, Mehl A, Hickel R, Klaiber B. The effect of working parameters on root substance removal using a piezoelectric ultrasonic scaler in vitro. J Clin Periodontol. 1998;25:158–163.
- Lea SC, Felver B, Landini G, Walmsley AD. Three-dimensional analyses of ultrasonic scaler oscillations. J Clin Periodontol. 2009;36:44–50.
- Lea SC, Landini G. Reconstruction of dental ultrasonic scaler 3D vibration patterns from phase-related data. Med Eng Phys. 2010;32:673–677.
- Lea SC, Landini G, Walmsley AD. The effect of wear on ultrasonic scaler tip displacement amplitude. J Clin Periodontol. 2006;33:37–41.
- Arabaci T, Cicek Y, Dilsiz A, Erdogan IY, Kose O, Kizilda IA. Influence of tip wear of piezoelectric ultrasonic scalers on root surface roughness at different working parameters. A profilometric and atomic force microscopy study. Int J Dent Hyg. 2013;11:69–74.
- Walmsley AD, Lea SC, Landini G, Moses AJ. Advances in power driven pocket/root instrumentation. J Clin Periodontol. 2008;35(Suppl 8):22–28.
- Rühling A, Bernhardt O, Kocher T. Subgingival debridement with a teflon-coated sonic scaler insert in comparison to conventional instruments and assessment of substance removal on extracted teeth. Quintessence Int. 2005;36:446–452.
From Dimensions of Dental Hygiene. January 2014;12(1):22–24,26–27.

