
Changing the Trajectories of Diabetes and Periodontitis
Oral health professionals who understand the interrelationship of the inflammatory burden shared by these two chronic diseases will be best prepared to provide customized treatment.
This course was published in the September 2017 issue and expires September 2020. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Discuss the association between diabetes and periodontal diseases.
- Identify the evidence-based statements that should guide treatment planning for patients at risk of or those who have diabetes.
- List the four categories of risk used in patient classification to help clinicians customize treatment.
Since Löe1 suggested that periodontitis should be the sixth complication of diabetes in 1993, scientific literature has abounded with evidence of this interrelationship2–43 and the unique role that oral health professionals play in diabetes screening.44–46 Performing medical screening in dental settings began more than 50 years ago when assessing patients for hypertension became a standard of care. Given the magnitude of the diabetes epidemic, screening people at risk for diabetes or identifying people with undiagnosed diabetes should no longer be optional during routine dental visits. Further, the substantial evidence on the interrelationship between diabetes and periodontal diseases compels clinicians to consider how customizing the care of patients in four different risk categories may change the trajectory of diabetes and/or periodontal diseases in an individual’s life.
Diabetes and periodontitis each contribute to the total inflammatory burden. However, in individuals who have both diabetes and periodontitis, this combination confers greater risk, as these interrelated chronic disease states act synergistically to amplify the total inflammatory burden. Importantly, when both diseases are present, the total inflammatory burden may be increased.
Traditionally, screening for diabetes and periodontitis has occurred in silos within the health care arena, highlighting the historical schism between dentistry and medicine. The medical community has largely been responsible for identifying people with diabetes and oral health professionals have been responsible for identifying individuals with periodontitis. However, the magnitude of the diabetes and periodontitis epidemics compels health care professionals to dismantle these silos in favor of bilateral point-of-care screening, referrals, and co-management of these chronic diseases. This requires physicians, nurses, and other health care providers to screen patients for periodontitis, and oral health professionals to screen patients for diabetes and prediabetes. Referral and medical-dental co-management of these cases is essential.
The epidemic of diabetes and prediabetes and the high prevalence of periodontitis pose challenges to health care delivery systems throughout the world. According to most recent reports, 387 million people worldwide have diabetes, about half of which are undiagnosed cases.47 By 2035, approximately 592 million people in the world will have diabetes. The prevalence of prediabetes is also dramatically increasing. By 2030, 470 million people across the globe are projected to have prediabetes.48 The prediabetic state of impaired glucose regulation affects 38% of United States adults.49
About 11% of the world’s population has severe periodontitis; however, this estimate varies according to geographic location. The prevalence and severity also increase with age and vary with ethnicity.50,51 This estimate only includes severe cases of periodontitis. In the US, about 47% of people age 30 and older have periodontitis, including mild, moderate, and severe cases.52 The prevalence of diabetes and periodontitis and the metabolic synergy created by their interrelationship compel health care professionals to incorporate progressive screening strategies that may change the trajectories of diabetes and periodontitis.
PRECEPTS FOR PATIENT CARE
More than 50 years ago, Belting et al53 reported that diabetes influenced the severity of periodontal diseases. Today, the plethora of research on this interrelationship supports a progressive intervention strategy to identity patients in various categories of diabetes and periodontitis who may benefit from customized care. The following statements—which have strong evidence bases—should guide treatment planning and maintenance of patients who either have or who may be at risk for diabetes and periodontitis.
- Type 1 and type 2 diabetes both increase the risk for periodontal disease pathogenesis.2–6
- Risk for deterioration of the periodontium, including clinical attachment loss (CAL), progression of periodontal probing depth, and alveolar bone loss (ABL), is increased when diabetes is poorly controlled.7–9 There may be an associated gradient, or dose-response relationship in so much as when glycemic control worsens, the greater the adverse effect diabetes will have on periodontal health.10
- Periodontal infection/inflammation is a risk factor for poor glycemic control and compromised diabetes management.8,11
- Risk for complications of diabetes, such as retinopathy, neuropathy, nephropathy, proteinuria, end-stage renal disease, and cardiovascular disease, is increased in people with periodontal diseases.12–20
- Chronic periodontal inflammation in people who do not have diabetes may increase the risk for developing type 2 diabetes.21–27
- The preponderance of evidence advises that the treatment of periodontal diseases may improve glycemic control; however, this is still inconclusive.28–37 A Cochrane Review reported that there is insufficient evidence to support the theory that improvement in glycemic control can be maintained 4 months following treatment using nonsurgical treatment modalities; however, treatment of periodontal disease is associated with improved glycemic control and about a 0.4% reduction in hemoglobin A1c (HbA1c).38 The report also suggested that maintaining clinical improvement in glycemic control beyond 6 months, can only be accomplished through ongoing professional periodontal treatment. In addition, the report noted that no one periodontal therapy is more effective than another in improving glycemic control in people with diabetes, and it is inconclusive whether adjunctive therapies improve periodontal treatment outcomes.
- Periodontal indices that trend negatively may suggest a patient has unidentified prediabetes.39 For example, by observing changes in periodontal tissues, undiagnosed abnormal fasting plasma glucose may be detected. The development of glucose intolerance is associated with periodontal pockets.40 Periodontitis may be associated with decreased beta-cell function and impaired glucose metabolism. These are characteristic of prediabetes.41–43 In the Oral Infections, Glucose Intolerance and Insulin Resistance Study, Demmer et al54 found that higher subgingival colonization levels of specific periodontal microorganisms are associated with prediabetes.

STRATIFYING RISK
Given the strong evidence that co-existing diabetes and periodontitis act together to amplify cumulative inflammatory burden, oral health professionals should identify and differentiate dental patients according to four risk categories. These patients may benefit from more comprehensive, profile-specific care, which may have the potential to avert or delay the onset of diabetes/periodontitis. A proposal on how these at-risk patient groups could be stratified and ideas on how care may be customized appear below.

*right-click Save Image As to download this photo.
Category 1: Patients with diabetes and no periodontitis. It’s important to ask patients with diabetes about their most recent HbA1c test, which measures an individual’s average blood sugar control over the past 2 months to 3 months. Maintaining blood sugar levels as close to normal, as safely as possible, is the goal in diabetes care.55 The risk for diabetes-related complications can be brought about by small changes in HbA1c. Maintaining HbA1c to below or around 7% reduces complications, including a decrease in the risk of periodontitis. The opposite is also true—increased blood glucose signals the potential for increased susceptibility to periodontitis. People with diabetes should go for HbA1c testing as soon as they are diagnosed and then 2 times to 4 times a year depending on how well their blood sugar is under control.
Oral health professionals need to monitor patients diagnosed with diabetes who do not have treating physicians. These individuals are at high risk for cardiovascular events and should be referred to a physician.56 Careful monitoring of periodontal status is essential in caring for dental patients with diabetes. Blood pressure should be tracked, including measuring patients’ blood pressure while seated at rest in the dental operatory. In addition, clinicians should educate patients with diabetes about increased risk for infections, including periodontal diseases, and consistently review HbA1c results and blood pressure and cholesterol levels. The status of a patient’s glycemic control and diabetes case management should be routinely documented, including the patient’s responses to questions in Table 1.

*right-click Save Image As to download this photo.
Category 2: Patients with diabetes who also have untreated periodontitis.Glycemic control may worsen when periodontitis remains untreated. When patients with diabetes are diagnosed with periodontitis, they need to be educated about the role of untreated periodontitis in disrupting glycemic control and subsequently increasing risk for complications of diabetes. Patients should be counseled that treatment of periodontitis may improve glycemic control, and, as a result, reduce insulin requirements. Patients should be encouraged to accept recommended periodontal treatment plans; for patients who refuse treatment, it’s important to advise their physicians of the impact untreated periodontitis may have on glycemic control. With results from patients’ last HbA1c tests, oral health professionals can monitor glycemic control against worsening periodontal indices (eg, bleeding on probing [BOP], increased probing depths). All members of the diabetes care team should be vigilant in looking for signs and symptoms of periodontitis.
*right-click Save Image As to download this photo.
Category 3: Patients with diabetes who also have periodontitis that has been treated. There is evidence that 3-month intervals of periodontal maintenance may be effective in sustaining periodontal health in most patients after initial treatment of periodontitis.57 However, patients with poorly controlled diabetes may have a more rapid recurrence of deep probing depths, and their long-term response to periodontal treatment may be compromised.58,59 Accordingly, results of the patient’s most recent HbA1c test may aid in determining why he or she had a suboptimal response to therapy. If periodontal indices—such as BOP and increased probing depths—do not improve, it may be best to move the patient back to active therapy and perhaps consult his or her physicians.60 After completion of periodontal therapy, patients with diabetes should be assigned periodontal maintenance intervals according to their response to treatment and glycemic control. Again, patients should be educated about how periodontal infection impacts glycemic control, and be encouraged to take responsibility for managing their diabetes and periodontal health. Clinicians should also assess risk factors for periodontitis, as these may influence the development of diabetic complications.60
*right-click Save Image As to download this photo.
Category 4: Patients who have been diagnosed with periodontitis and are systemically healthy. Periodontitis increases the risk for impaired glucose intolerance. It is through this biological cascade that chronic periodontal inflammation may increase the risk for diabetes. As such, oral health professionals need to routinely screen patients with periodontitis for diabetes or prediabetes. Patients should be educated about the role of periodontal infection in increasing the risk for diabetes and the importance of periodontal disease treatment. Borrell et al61 reported a novel model for predicting the onset of diabetes. Dental patient subjects who self-reported a constellation of risk factors that included a family history of diabetes, hypertension, and high cholesterol, and also presented with clinical evidence of periodontal disease had a probability of 27% to 53% of developing diabetes.61 Saito et al40 found that subjects who developed impaired glucose tolerance were significantly more likely to have deep periodontal pockets.
Evolving evidence suggests there is a correlation between the progression of periodontitis and severity of obesity, and an association between metabolic syndrome (MetS) and periodontal disease.62 Research findings in this area are consistent enough that it has prompted some investigators to propose that periodontitis should be considered a component of MetS. Oral health professionals are uniquely positioned to identify patients who present with the appearance of central adiposity—or apple-shaped physique—a strong predictor of MetS.62
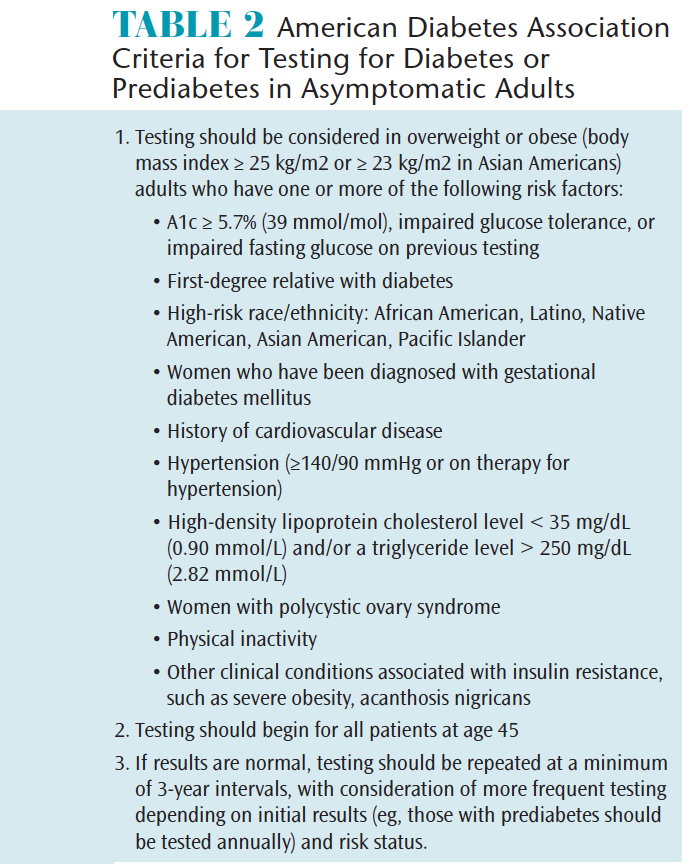
Clinicians should be diligent in recognizing patients who present with the classic signs and symptoms of diabetes (eg, increased thirst and/or dry mouth, increased hunger, unexplained fatigue, increased urination, unexpected weight loss, blurred vision, and slow and/or poor healing) and refer them to physicians as soon as possible. However, a significant number of individuals with diabetes do not yet manifest the classic signs and symptoms of diabetes. Every year the American Diabetes Association publishes updated criteria for identifying these asymptomatic adults who should be tested for diabetes or prediabetes. Table 2 provides criteria from the organization’s 2017 guidelines.63
The American Diabetes Association’s most recent guidelines for diabetes care emphasize the importance of screening for prediabetes with assessment tools or informal assessment of risk factors and performing diagnostic testing when appropriate.63 Early intervention—such as lifestyle modification—may reduce progression from prediabetes to diabetes.64 Usually, no symptoms are present during the stage of insulin resistance and prediabetes. Consequently, people often have these conditions for years without knowing it. Oral health professionals are uniquely positioned to identify patients who have prediabetes, make referrals to patients’ physicians, and intervene with patient education targeting lifestyle modification that may avert or delay the onset of diabetes.
Herman et al46 demonstrated a simple, yet powerful tool for identifying patients at risk for prediabetes. The 1-page questionnaire includes questions about gender; history of hypertension, dyslipidemia, and lost teeth, and self-reported body mass index (BMI). They found that these criteria and BMI ≥ 35 kg/m2, with or without performing a finger stick to quantify random capillary glucose, could identify subjects with prediabetes. These researchers estimated that 30% of patients who are age 30 or older may have prediabetes. Many of these individuals see oral health professionals on a routine basis—providing an excellent opportunity to screen patients for prediabetes.46
Javed et al65 reported on changes in various periodontal parameters that may be sensitive enough to identify people with prediabetes. They found that people with poorly controlled prediabetes may be more susceptible to periodontal destruction as evidenced by increased BOP, probing depths, CAL, and marginal ABL. Vigilant oral health professionals can recognize these changes and may be the earliest health care provider to recognize prediabetes.
Rao et al66 discovered that screening for high blood glucose could be done by sampling blood from gingival crevicular fluid during routine periodontal examination in populations with an unknown history of diabetes. They reported that although the technique is safe and comfortable, obtaining blood samples from gingival crevicular fluid is technique-sensitive, making its viability in every day patient care questionable. Koneru et al67 found that blood acquired during periodontal probing may be a dependable way to screen for diabetes in people who have periodontal diseases.
CONCLUSION
Extensive evidence collected over the past 50 years has established that as individual chronic disease states, diabetes and periodontitis amplify cumulative inflammatory burden. However, when these chronic diseases co-exist, the inflammatory burden is synergistically increased, and treatment and management of these diseases—which are often complex—can be compromised if oral health professionals fail to recognize this interrelationship. Clinicians who progressively integrate this knowledge into clinical care may be the most promising in averting the trajectory of diabetes and periodontitis—both of which are epidemic in magnitude. One strategy is categorizing patients in four different risk groups: patients with diabetes and no periodontitis; patients with diabetes who also have untreated periodontitis; patients with diabetes who also have periodontitis that has been treated; and patients who have been diagnosed with periodontitis who are systemically healthy. By stratifying dental patients in these risk groups, oral health professionals can more systematically customize care, which may translate into more successful clinical outcomes. Cases like these will benefit from dental-medical collaboration and more patient-centric treatment planning and preventive protocols.
Future research in support of a risk-prediction model that provides for identification of dysglycemia, even before prediabetes is present, will provide the opportunity for oral health professionals to intervene with patient education and counseling in lifestyle modification that may avert or delay the onset of diabetes. Not only will this remap the trajectory of diabetes and periodontitis in an individual’s life, but it will enhance the role of oral health professionals on the diabetes care team.
REFERENCES
- Löe H Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care. 1993;16:329–334.
- Salvi GE, Carollo-Bittel B, Lang NP. Effects of diabetes mellitus on periodontal and peri-implant conditions: update on associations and risks. J Clin Periodontol. 2008;35(8 Suppl):398–409.
- Chavarry NG, Vettore MV, Sansone C, Sheiham A. The relationship between diabetes mellitus and destructive periodontal disease: a meta-analysis. Oral Health Prev Dent. 2009;7:107–127.
- Khader YS, Dauod AS, El-Qaderi SS, Alkafajei A, Batayha WQ. Periodontal status of diabetics compared with nondiabetics: a meta-analysis. J Diabetes Complications. 2006;20:59–68.
- Tsai C, Hayes C, Taylor GW. Glycemic control of type 2 diabetes and severe periodontal disease in the US adult population. Community Dent Oral Epidemiol. 2002;30:182–192.
- Mealey BL, Ocampo GL. Diabetes mellitus and periodontal disease. Periodontol 2000. 2007;44:127–153.
- Bandyopadhyay D, Marlow M, Fernandes J, Leite R. Periodontal disease progression and glycaemic control among Gullah African Americans with type-2 diabetes. J Clin Periodontol. 2010;37:501–509.
- Taylor GW, Burt BA, Becker MP, Genco RJ, Shlossman M. Glycemic control and alveolar bone loss progression in type 2 diabetes. Ann Periodontol. 1998;3:30–39.
- Collin HL, Uusitupa M, Niskanen L, et al. Periodontal findings in elderly patients with non-insulin dependent diabetes mellitus. J Periodontol. 1998;69:962–966.
- Taylor G, Borgnakke W. Periodontal disease: associations with diabetes, glycemic control and complications. Oral Dis. 2008;14:191–203.
- Taylor GW, Burt BA, Becker MP, et al. Severe periodontitis and risk for poor glycemic control in patients with non-insulin-dependent diabetes mellitus. J Periodontol. 1996;67(10 Suppl):1085–1093.
- Banthia R, Raje S, Banthia P, Saral SK, Singh P, Gupta S. Evaluation of the association between periodontal disease and diabetic retinopathy. Gen Dent. 2014;62:e28–32.
- Karjalainen KM, Knuuttila ML, von Dickhoff KJ. Association of the severity of periodontal disease with organ complications in type 1 diabetic patients. J Periodontol. 1994;65:1067–1072.
- Moore P, Weyant R, Mongelluzzo M, Myers D, Rossies K, Guggenheimer J, et al. Type 1 Diabetes Mellitus and Oral Health: Assessment of Tooth Loss and Edentulism. Journal of Public Health Dentistry 1998;58(2):135-142.
- Moore P, Weyant R, Mongelluzzo M, et al. Type 1 diabetes mellitus and oral health: assessment of periodontal disease. J Periodontol. 1999;70:409–417.
- Thorstensson H, Kuylenstierna J, Hugoson A. Medical status and complications in relation to periodontal disease experience in insulin-dependent diabetics. J Clin Periodontol. 1996;23(3 Pt 1):194–202.
- Shultis WA, Weil EJ, Looker HC, et al. Effect of periodontitis on overt nephropathy and end-stage renal disease in type 2 diabetes. Diabetes Care. 2007;30:306–311
- Saremi A, Nelson RG, Tulloch-Reid M, Hanson RL, Sievers ML, Taylor GW, et al. Periodontal disease and mortality in type 2 diabetes. Diabetes Care. 2005;28:27–32.
- Patel A, Chalmers J, Poulter N. Advance: action in diabetes and vascular disease. J Hum Hypertens. 2005;19(Suppl 1):S27–S32.
- Li Q, Chalmers J, Czernichow S, et al. Oral disease and subsequent cardiovascular disease in people with type 2 diabetes: a prospective cohort study based on the action in diabetes and vascular disease: Preterax and Diamicron modified-release controlled evaluation (Advance) trial. Diabetologia. 2010 Nov;53(11):2320-2327.
- Demmer RT, Jacobs DR Jr, Desvarieux M. Periodontal disease and incident type 2 diabetes: results from the First National Health and Nutrition Examination Survey and its epidemiologic follow-up study. Diabetes Care. 2008;31:1373–1379.
- Wolff RE, Wolff LF, Michalowicz BS. A pilot study of glycosylated hemoglobin levels in periodontitis cases and healthy controls. J Periodontol. 2009;80:1057–1061.
- Demmer RT, Desvarieux M, Holtfreter B, et al. Periodontal status and A1C change: longitudinal results from the study of health in Pomerania (SHIP). Diabetes Care. 2010;33:1037–1043.
- Santos Tunes R, Foss-Freitas MC, Nogueira-Filho Gda R. Impact of periodontitis on the diabetes-related inflammatory status. J Can Dent Assoc. 2010;76:a35.
- Ide R, Hoshuyama T, Wilson D, Takahashi K, Higashi T. Periodontal disease and incident diabetes: a seven-year study. J Dent Res. 2011;90:41–46.
- Yu N, Barros S, Zhang S, Moss K, Phillips S, Offenbacher S. Insulin response genes in different stages of periodontal disease. J Dent Res. 2015;94(9 Suppl):194S–200S.
- Borgnakke WS, Ylostalo PV, Taylor GW, Genco RJ. Effect of periodontal disease on diabetes: systematic review of epidemiologic observational evidence. J Clin Periodontol. 2013;40(Suppl 14):S135–52.
- Corbella S, Francetti L, Taschieri S, De Siena F, Fabbro MD. Effect of periodontal treatment on glycemic control of patients with diabetes: A systematic review and meta-analysis. J Diabetes Investig. 2013;4:502–509.
- Engebretson S. Periodontal disease and glycemic control in diabetics. Evid Based Dent. 2014;15:93–94.
- Gurav A. Periodontal therapy—an adjuvant for glycemic control. Diabetes Metab Syndr. 2012;6:218–223.
- Li Q, Hao S, Fang J, Xie J, Kong XH, Yang JX. Effect of non-surgical periodontal treatment on glycemic control of patients with diabetes: a meta-analysis of randomized controlled trials. Trials. 2015;16:291–292.
- Wang X, Han X, Guo X, Luo X, Wang D. The effect of periodontal treatment on hemoglobin a1c levels of diabetic patients: a systematic review and meta-analysis. PLoS One. 2014;9:e108412.
- Preshaw PM, Alba AL, Herrera D, , et al. Periodontitis and diabetes: a two-way relationship. Diabetologia. 2012;55:21–31.
- Mauri-Obradors E, Jané-Salas E, Sabater-Recolons M, Vinas M, López-López J. Effect of nonsurgical periodontal treatment on glycosylated hemoglobin in diabetic patients: a systematic review. Odontology. 2015;103:301–313.
- Wang TF, Jen IA, Chou C, Lei YP. Effects of periodontal therapy on metabolic control in patients with type 2 diabetes mellitus and periodontal disease: a meta-analysis. Medicine (Baltimore). 2014;93:e292.
- Jones J, Miller D, Wehler C, et al. Does periodontal care improve glycemic control? The Department of Veterans Affairs Dental Diabetes Study. J Clin Periodontol. 2007;34:46–52.
- Darre L, Vergnes J, Gourdy P, Sixou M. Efficacy of periodontal treatment on glycaemic control in diabetic patients: A meta-analysis of interventional studies. Diabetes Metab. 2008;34:497–506.
- Simpson TC, Weldon JC, Worthington HV, et al. Treatment of periodontal disease for glycaemic control in people with diabetes mellitus. Cochrane Database Syst Rev. 2015;11:CD004714.
- Lamster I, Cheng B, Burkett S, Lalla E. Periodontal findings in individuals with newly identified pre-diabetes or diabetes mellitus. J Clin Periodontol. 2014;41:1055–1060.
- Saito T, Shimazaki Y, Kiyohara Y, et al. The severity of periodontal disease is associated with the development of glucose intolerance in non-diabetics: the Hisayama study. J Dent Res. 2004;83:485–490.
- Islam SA, Seo M, Lee YS, Moon SS. Association of periodontitis with insulin resistance, beta-cell function, and impaired fasting glucose before onset of diabetes. Endocr J. 2015;62:981–989.
- Timonen P, Saxlin T, Knuuttila M, et al. Role of insulin sensitivity and beta cell function in the development of periodontal disease in adults without diabetes. J Clin Periodontol. 2013;40:1079–1086.
- Arora N, Papapanou P, Rosenbaum M, Jacobs D, Desvarieux M, Demmer R. Periodontal infection, impaired fasting glucose and impaired glucose tolerance: results from the Continuous National Health and Nutrition Examination Survey 2009-2010 . J Clin Periodontol. 2014;41:643–652.
- Strauss SM, Alfano MC, Shelley D, Fulmer T. Identifying unaddressed systemic health conditions at dental visits: patients who visited dental practices but not general health care providers in 2008. Am J Public Health. 2012;102:253–255.
- Strauss SM, Russell S, Wheeler A, Norman R, Borrell LN, Rindskopf D. The dental office visit as a potential opportunity for diabetes screening: an analysis using NHANES 2003-2004 data. J Public Health Dent. 2010;70:156–162.
- Herman WH, Taylor GW, Jacobson JJ, Burke R, Brown MB. Screening for prediabetes and type 2 diabetes in dental offices. J Public Health Dent. 2015;75:175182.
- International Diabetes Federation. IDF Diabetes Atlas Sixth Edition Poster Update 2014. Available at: idf.org/sites/default/files/Atlas-poster-2014_EN.pdf. Accessed August 23, 2017.
- IDF Diabetes Atlas. 5th ed. International Diabetes Federation. Brussels: Belgium; 2011.
- Go AS, Mozaffarian D, Roger VL, et al. Heart Disease and Stroke Statistics- 2013 update: A Report from the American Heart Association. Circulation. 2013;127:e6–e245.
- Richards D. Review finds that severe periodontitis affects 11% of the world population. Evid Based Dent. 2014;15:70–71.
- Marcenes W, Kassebaum N, Bernabé E, et al. Global burden of oral conditions in 1990-2010: a systematic analysis. J Dent Res. 2013;92:592–597.
- Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ, CDC Periodontal Disease Surveillance workgroup. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91:914–920.
- Belting C, Hiniker J, Dummett C. Influence of diabetes mellitus on the severity of periodontal disease. J Periodontol. 1964;35:476–480.
- Demmer RT, Jacobs DR, Singh R, Rosenbaum M, Papapanou PN, Desvarieux M. Periodontal bacteria and prediabetes prevalence in ORIGINS: the oral infections, glucose intolerance, and insuline resistance study. J Dent Res. 2015;94(9 Suppl):201S–2011S.
- Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986.
- Hein C. Proceedings and consensus opinion from the global oral and systemic health summit: Present evidence and future directions. Grand Rounds in Oral-Systemic Medicine. Available at: http://www.maineoralhealthcoalition.org/docs/gr_supplement_feb07.pdf. Accessed August 23, 2017.
- American Academy of Periodontology. Position paper: periodontal maintenance. J Periodontol. 2003;74:1395–1401.
- Tervonen T, Karjalainen K. Periodontal disease related to diabetic status. A pilot study of the response to periodontal therapy in type 1 diabetes. J Clin Periodontol. 1997;24:505–510.
- Mealey BL, Oates TW. AAP-commissioned review: diabetes and periodontal diseases. J Periodontol. 2006;77:1289–1303.
- American Academy of Periodontology. Parameter on systemic conditions affected by periodontal diseases. J Periodontol. 2000;71:880–883.
- Borrell LN, Kunzel C, Lamster I, Lalla E. Diabetes in the dental office: using NHANES III to estimate the probability of undiagnosed disease. J Periodontal Res. 2007;42:559–565.
- Hein C, Batista H. Obesity and cumulative inflammatory burden: a valuable risk assessment parameter in caring for dental patients. J Evid Based Dent Pract. 2014;14(Suppl):17–26.
- American Diabetes Association. Standards of medical care in diabetes—2017. J Clin Appl Res Educ. 2017;40(Suppl 1): S1–S135
- Phillips LS, Ratner RE, Buse JB, Kahn SE. We can change the natural history of type 2 diabetes. Diabetes Care. 2014;37:2668–2676.
- Javed F, Thafeed Alghamdi AS, Mikami T, et al. Effect of glycemic control on self-perceived oral health, periodontal parameters, and alveolar bone loss among patients with prediabetes. J Periodontol. 2014;85:234–241.
- Rao MV, Reddy MV, Sunder SS, Kolasani B, Kiranmai G, Kumar KR. In-dental office screening for diabetes mellitus using gingival crevicular blood. J Int Soc Prev Comm Dent. 2014;4(Suppl 3):S161–S165.
- Koneru S, Tanikonda R. Reliability of gingival blood sample to screen diabetes in dental hospital. Int J Prev Med. 2015;6:23.
Featured photo by DOLGACHOV/E+/GETTY IMAGES PLUS
From Dimensions of Dental Hygiene. September 2017;15(9):40-43.



