 WILDPIXEL/ISTOCK/THINKSTOCK
WILDPIXEL/ISTOCK/THINKSTOCK
Caring For Patients With Spina Bifida
This complex neural tube defect causes a variety of impairments that can impact the delivery of dental treatment.
This course was published in the January 2017 issue and expires January 2020. The author has no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Discuss the prevalence, incidence, and ramifications of spina bifida.
- Define the risks and protective factors associated with the development of this neural tube defect.
- Differentiate characteristics between various types of spina bifida.
- List common impairments and complications associated with spina bifida.
- Identify oral health care considerations for patients with spina bifida.
The United States Centers for Disease Control and Prevention’s (CDC) National Spina Bifida Patient Registry and Surveillance System estimates the national prevalence of spina bifida at 166,000 individuals.4 Researchers estimate the national annual incidence of spina bifida to be approximately 1,500 live births.4
There are significant social and economic costs for individuals with this neural tube defect. Multiple studies have tracked national expenditure trends in populations with spina bifida. Children with spina bifida had an approximate 13-fold increase in health care expenditures compared to children without neural tube defects, whereas adults with spina bifida experienced a three-fold to six-fold increase in health care expenditures compared to adults without spina bifida.5 The medical care and surgical costs for all individuals with spina bifida exceed $200 million annually, with the lifetime cost of care for each child born with spina bifida estimated at more than half a million dollars.5
Diagnostic screenings for the presence of spina bifida begins in the first trimester of pregnancy at 28 days of gestation. To detect spina bifida, the mother’s maternal serum alpha-fetoprotein (MSAFP) levels can be measured, a thorough ultrasound examination can be performed, or an amniocentesis can be conducted.3
Multiple risk factors are associated with the development of spina bifida: familial history of neural tube defects; mother’s consumption of anticonvulsant medication; and mother’s systemic health status, such as presence of obesity and/or diabetes mellitus type 2.4Clinical and public health studies have indicated that the proper intake of folic acid is the most significant preventive method in reducing the risk of spina bifida.3 Folic acid is a water soluble B-9 vitamin that is available in supplements or through dietary sources, such as asparagus, avocado, beans, milk, orange juice, salmon, soybeans, and spinach.6 In 1992, the US Public Health Service published the mean recommended dosage to minimize risk for pregnancies with spina bifida at 0.4 mg (400 mcg).7 The CDC estimates that approximately 50% of all pregnancies in the US are unplanned; therefore, it is important for all childbearing age women to take the recommended dosage of folic acid daily.4 Women at increased risk, such as mothers with affected children, typically require a 10-fold prescriptive dosage of 4 mg folic acid per day at least 1 month to 3 months before conception and continuing through the first trimester of pregnancy to minimize risk for neural tube defect.8
Research has been conducted on the polymorphic mutational impact of the methylenetetrahydrofolate reductase (MTHFR) gene in the incidence of spina bifida.9 The polymorphic mutation in the MTHFR gene modifies the innate ability of the subsequent MTHFR enzyme to process folate in the body.9 Further research is needed on the role of the MTHFR gene and enzyme on the incidence of neural tube defects.
TYPOLOGY
There are four types of spina bifida: occulta; closed neural tube defect; meningocele; and myelomeningocele, with the closed neural tube defect being the most rare.3,4 Occulta is the mildest and most common form, in which the vertebrae are malformed.3 Occulta is present in approximately 10% to 20% of the affected population, rarely causes disability, and is typically asymptomatic.3 Closed neural tube defect consists of a diverse group of vertebral defects marked by malformation of fat, bone, or membranes, and can cause neurogenic bowel and bladder.3
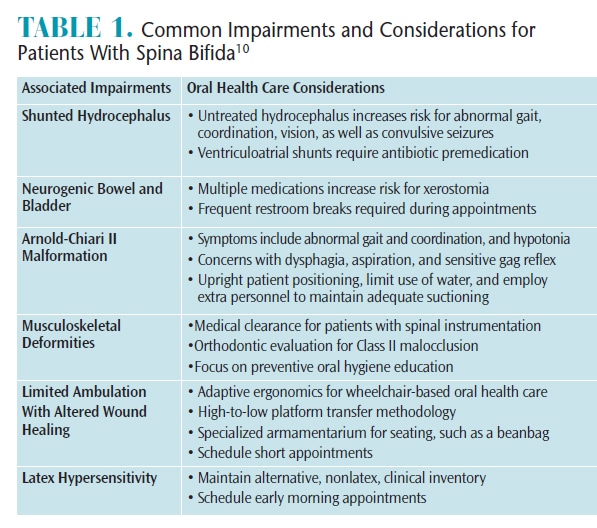 Meningocele and myelomeningocele manifest with a physiological protrusion through an abnormal vertebral opening. In myelomeningocele, however, the spinal cord and neural elements in the physiological protrusion are exposed and vulnerable to symptomatic paralysis.3 Table 1 outlines the commonly associated impairments and considerations in patients with spina bifida.10
Meningocele and myelomeningocele manifest with a physiological protrusion through an abnormal vertebral opening. In myelomeningocele, however, the spinal cord and neural elements in the physiological protrusion are exposed and vulnerable to symptomatic paralysis.3 Table 1 outlines the commonly associated impairments and considerations in patients with spina bifida.10
RELATED IMPAIRMENTS
Hydrocephalus, or fluid in the brain, is caused by excessive accumulation of cerebral spinal fluid (CSF), resulting in increased pressure in the ventricular environment.11 Approximately 80% of patients with spina bifida experience hydrocephalus.6 An excessive accumulation of CSF presents as an abnormally rapid increase in cranial circumference or an unusually large head size.12 The abnormal widening of the craniofacial structure creates potentially harmful pressure on the cranial meninges, increasing risk for abnormal gait, altered coordination, visual impairment, developmental delay, and/or convulsive seizures.11 The typical medical protocol for hydrocephalus is the surgical placement of a biocompatible shunt to redirect excessive cerebral fluid from the ventricles into alternative vital organs for systemic excretion.10 Artificial shunts placed for the management of hydrocephalus include the ventriculoperitoneal (VP) shunt, ventriculoatrial shunt, ventriculopleural shunt, or the ventriculocholecyst shunt.12 The VP shunt is the most common.
The American Academy of Pediatric Dentistry and the American Heart Association recognize that patients with certain types of complex medical conditions are at an increased risk for bacteremia-induced infections, such as infective endocarditis.13 Patients with shunted hydrocephalus that have a ventriculoatrial shunt, ventriculocardiac shunt, or ventriculovenus shunt to drain excessive CSF are at a significantly increased risk for bacteremia-induced infections. As such, they should be prescribed prophylactic antibiotic premedication for invasive dental procedures, as should those at risk for infective endocarditis.13 Oral health care procedures that engage in “manipulation of gingival tissues, periapical regions of teeth, or perforation of oral mucosa” are invasive and susceptible to increasing the risk for infective endocarditis.13 The use of prophylactic antibiotic premedication decreases but does not eliminate the risk for bacterial-induced infections.13 Clinicians should be judicious and selective in prescribing antibiotics based on the individual needs of the patient to minimize risk for antibiotic resistance and allergic sensitization.
Patients with impaired autonomic nerve function can manifest with neurogenic bowel and bladder.11 The permanent nerve damage secondary to spina bifida can result in interrupted voluntary communication between the sphincter muscles, spinal column, and bowel/bladder function.14 Unfortunately, these patients experience both frequent urinary tract infections and/or frequent fecal impactions. The typical medical management of neurogenic bowel and bladder includes intermittent catheterization; urinary diversion, such as vesicostomy or bladder augmentation; and pharmaceutical interventions.4
There are multiple oral health considerations for populations affected with neurogenic bowel and bladder, including attentiveness to scheduling appointments, provider sensitivity to patients’ needs to use the restroom, and xerostomia management.14 Patients with neurogenic bowel and bladder should be encouraged to use the restroom before the appointment and clinicians should frequently suggest restroom breaks during lengthy appointments.14 Oral health professionals should suggest methods to mitigate decreased salivary flow, such as using over-the-counter glycerin-based mouthrinses and toothpastes with a neutral pH, saliva substitutes, and oral lubricants.14 Patients with xerostomia are also at an increased risk for dental caries and, therefore, need close monitoring and, if indicated, topical fluoride therapy.14
Some patients with spina bifida also manifest with the Arnold-Chiari II malformation complex. This presents as an inferior migration of the cerebellum directed proximally toward the foramen magnum; thus, the cerebellum’s depth of protrusion through the foramen magnum categorizes the stage of Arnold-Chiari malformation complex.3 Radiologists can detect the depth of the malformation through magnetic resonance imaging or computed tomography diagnostic imaging. The second stage of the complex, known as Arnold-Chiari II malformation, occurs when the cerebellar tonsils herniate through the foramen magnum.3 Approximately one in every 1,000 live births and one-third of patients with myelomeningocele have Arnold-Chiari II malformation.3,10 Patients can be asymptomatic; therefore, surgical intervention remains deferred until the patient is symptomatic.3 If the patient is symptomatic, then a surgical posterior fossa decompression intervention alleviates pressure at the compression site.3 The common signs and symptoms of Arnold-Chiari II malformation include abnormal gait, altered coordination, irregular respirations, dysphagia, increased aspiration risk, hypersensitive gag reflex, and hypotonia in the arms.6 The development of symptoms varies on the typology and severity of the spina bifida.10
Oral health care implications for patients with Arnold-Chiari II malformation include preliminary evaluation of a patient’s gait and coordination to assess increased risk for orofacial trauma secondary to falling.14 Patients with Arnold-Chiari II malformation should be positioned upright to minimize concerns with airway obstruction and dysphagia.14 Furthermore, oral health professionals should have extra personnel to assist with suctioning during procedures that use water to minimize the risk of aspiration.14 Clinicians can employ alternative approaches to provide customized care to patients with special health care needs, such as interchanging between wet and dry gauzes to cleanse the oral cavity during procedures instead of spraying the air/water syringe and suctioning with the saliva ejector if patients experience dysphagia and are at an increased risk for aspiration.
Many patients with spina bifida have orthopedic concerns such as clubfoot, dislocated hips, spinal dysplasia, and atypical musculoskeletal contractures.6 An estimated 90% of patients with a neural tube defect experience musculoskeletal deformities, such as scoliosis, kyphosis, or kyphoscoliosis.4 Patients with significant abnormal spinal curvatures undergo surgical rodding procedures that attempt to align the spinal column to a near-normal curvature.14 Some orthopedic surgeons require patients to obtain medical clearances from other clinicians, including the patient’s oral health professional, prior to spinal instrumentation. As such, patients who are candidates for spinal surgery need to complete oral health care treatment as soon as possible.14 Oral health professionals need to maintain communication with the patient and his/her orthopedic surgeon regarding the medical necessity for prophylactic antibiotic premedication for routine and invasive dental procedures post-spinal instrumentation.14
Patients with spina bifida are at increased risk for orofacial musculoskeletal concerns, such as Angles Class II malocclusions.15Patients with malocclusions and crowding have difficulty maintaining adequate oral hygiene and are at an increased risk for developing both dental caries and periodontal diseases.15 Patients with complex malocclusions may want to see an orthodontist for therapeutic realignment. Oral health professionals need to help patients minimize their incidence of oral diseases by stressing oral hygiene education, increasing preventive dental hygiene visits, placing dental sealants, frequently applying topical fluoride, and providing referrals to dental specialists, as necessary.
Patients with spina bifida are at an increased risk for a compromised vertebral column; thus, affected patients often experience a permanent altered sensory function.4 Most patients with spina bifida are limited in ambulation and are likely to use assistive devices, such as wheelchairs.14 There are multiple oral health care considerations for patients with limited ambulation and insensate skin. Clinicians need to identify if the patient, based on comfort and safety, should remain in the wheelchair or transfer to the dental chair for treatment. If the patient remains in the wheelchair, then oral health professionals will need to use adaptive ergonomics to complete the necessary treatment. Alternatively, if a patient transfer scenario is indicated, clinicians need to ensure that the patient’s receiving platform is at a lower height than the initial platform to minimize risk for patient injury. For example, if a patient transfer is from wheelchair to dental chair, the clinician should lower the dental chair so that the patient transfer is from a higher to lower plane. Conversely, if a patient transfer is from dental chair to wheelchair, then the clinician should raise the dental chair slightly so that the patient transfer is from a higher to lower plane. Additional resources, such as extra personnel and patient transfer boards, can assist the patient during transfers.
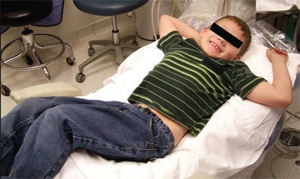
Patients with impaired sensory function are at increased risk for developing sores, calluses, blisters, and bruises. Due to localized spatial areas of insensate skin, patients with spina bifida are likely to be unaware of their pressure-based wound.4 Affected dental patients can benefit from the use of an intermediary device placed between the patient and the firm dental chair, such as a soft beanbag (Figure 1). A beanbag adapts to the patient’s musculoskeletal structure and redistributes the bodily pressures. Patients placed on soft beanbags are seated both on adaptive, more secure structures, reducing their risk for pressure wounds.14 Oral health professionals should ideally schedule short appointments to minimize these concerns.
LATEX SENSITIVITY
Patients with spina bifida are at increased risk of latex sensitivity.4 An estimated 70% of patients with spina bifida exhibit physiological symptoms such as pruritus, erythematous skin, itchy/watery eyes, dyspnea, wheezing, and slurred speech, when exposed to latex.16The exact etiology for latex sensitivity in patients with spina bifida is idiopathic; however, many sources trace the latex sensitivity to frequent exposures during both post-natal medical and surgical interventions.17 Studies have also demonstrated that latex sensitivities are associated with allergic reactions to bananas, strawberries, and kiwi.18 Many health care institutions have transitioned to nonlatex products when treating patients with spina bifida to mitigate concerns with sensitivity. Oral health professionals need to note that many products used in dentistry may contain latex, including, but not limited to adhesive bandages; blood pressure cuffs; personal protective equipment, such as gloves and masks; nitrous oxide reservoir bag; orthodontic bands/elastics; rubber cup polish; rubber dams; and saliva ejectors.18 An adequate supply of nonlatex alternatives for commonly used armamentarium should be maintained. Early morning clinical appointments are ideal for patients with latex sensitivity to minimize aerosol exposure.18
CONCLUSION
Patients with special health care needs, like spina bifida, are a particularly vulnerable and underserved population.19 Oral health professionals are valuable members of an interdisciplinary health care team dedicated to improving the overall health of these patients. Spina bifida is a complex neural tube defect with multiple associated impairments and considerations. Proactive oral health professionals need to ensure their teams are educated and clinically adaptive to the special health care needs of patients with spina bifida.
REFERENCES
- Fletcher JM, Brei TJ. Introduction: spina bifida–a multidisciplinary perspective. Dev Disabil Res Rev. 2010;16:1–5.
- Medline Plus. Neural Tube Defects. Available at: nlm.nih.gov/medlineplus/neuraltubedefects.html. December 28, 2016.
- National Institute of Neurological Disorders and Stroke. Spina Bifida Fact Sheet. Available at: ninds.nih.gov/disorders/spina_bifida/detail_spina_bifida.htm. Accessed December 27, 2016.
- Centers for Disease Control and Prevention. Spina Bifida. Available at: cdc.gov/ncbddd/ spinabifida/data.html. Accessed December 27, 2016.
- Ouyang L, Grosse S, Armour B, Waitzman N. Health care expenditures of children and adults with spina bifida in a privately insured U.S. population. Birth Defects Res A Clin Mol Teratol. 2007;79:552–528.
- Spina Bifida Association. Overview. Available at: spinabifidaassociation.org. Accessed December 27, 2016.
- Recommendations for the Use of Folic Acid to Reduce the Number of Cases of Spina Bifida and Other Neural Tube Defects. MMWR Recomm Rep. 1992;41(RR-14):1–7.
- Folic acid for the prevention of neural tube defects. American Academy of Pediatrics. Committee on Genetics. Pediatrics. 1999;104:325–327.
- US National Library of Medicine. MTHFR Gene. Available at: ghr.nlm.nih.gov/gene/MTHFR. Accessed December 27, 2016.
- Kabani F, Anderson M. Treating children with spina bifida. Dimensions of Dental Hygiene. 2012;10(4):52–57.
- March of Dimes. Spina Bifida. Available at: marchofdimes.org/baby/spina-bifida.aspx#. Accessed December 27, 2016.
- Mayo Clinic. Diseases and Conditions Spina Bifida. Available at: mayoclinic.org/diseases-conditions/spina-bifida/basics/definition/CON-20035356?=1&p=1. Accessed December 27, 2016.
- American Academy on Pediatric Dentistry Clinical Affairs Committee.; American Academy on Pediatric Dentistry Council on Clinical Affairs. Guideline on antibiotic prophylaxis for dental patients at risk for infection. Pediatr Dent. 2008-2009;30(7 Suppl):215–218.
- Kabani F. Special care for special needs populations: spina bifida. Presented at Collin County Dental Hygiene Society; October 24, 2015; McKinney, Texas.
- McGuire S. Presentation and Management of Patients at Texas Scottish Rite Hospital for Children: Spina Bifida. Presented at Special Care Dentistry Conference; April 20, 2013; New Orleans.
- American Academy of Allergy, Asthma, & Immunology. Latex Allergy. Available at: aaaai.org/conditions-and-treatments/allergies/latex-allergy.aspx. Accessed December 27, 2016.
- American Latex Allergy Association. AANA Latex Protocol. Available at: latexallergyresources.org/ articles/aana-latex-protocol. Accessed December 27, 2016.
- Spina Bifida Association. Latex in the Hospital Environment. Available at: spinabifidaassociation. org/project/latex-hospital. Accessed December 27, 2016.
- Lo A, Polsek D, Sidhu S. Estimating the burden of neural tube defects in low-and middle-income countries. J Glob Health. 2014;4:010402.
From Dimensions of Dental Hygiene. January 2017;15(1):48-51.



