
Balance the Scales
Calcium phosphate technologies may support the remineralization process, thereby helping to ensure the integrity of the tooth surface in the face of mineral loss.
Maintaining a balance between the remineralization and demineralization processes in the oral cavity is the basis of dental health. Once the scale weighs too heavily toward the demineralization side (loss of mineral), the tooth structure will begin to erode. The ongoing process of losing minerals (demineralization) and gaining minerals (remineralization) naturally occurs in the oral cavity throughout the day. For this reason, creating an oral environment conducive to remineralization is key.
Dental biofilm also plays a role in this process. When fermentable carbohydrates are consumed, the microorganisms in biofilm convert the carbohydrates into organic acids, such as lactic acid (Figure 1), which lowers the pH in the mouth. This is the start of the demineralization process, which can lead to softening of the enamel and caries.1 When the pH in the oral cavity is increased through saliva’s natural buffering system, the absence of fermentable carbohydrates, and the availability of minerals, the scale tips toward remineralization, and the integrity of the tooth surface is maintained. By sustaining conditions favorable to the remineralization process in the oral cavity, replacement of lost dental enamel is feasible.2
CONSEQUENCES
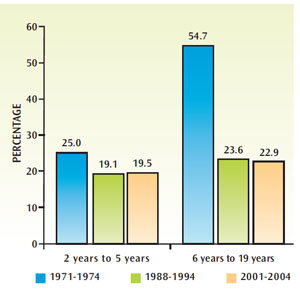
Though the prevalence of dental caries has declined among certain populations, tooth decay remains an oral health concern. Although rates of untreated dental caries have decreased significantly since the early 1970s, they have remained steady since the late 1980s and early 1990s (Figure 2). Between 2001 and 2004, 19.5% of children age 2 to 5 and 22.9% of children age 6 to 19 experienced untreated tooth decay.3 The prevalence is much higher among minority populations, especially Mexican-Americans, and those of low socioeconomic status.3 Caries is preventable and much research has been done into risk assessment, early detection methods, and noninvasive treatment options. Many strategies are available to tip the scales toward remineralization—from topical fluoride use to reducing exposure to fermentable carbohydrates. Table 1 provides a summary of appropriate adjunct remineralization therapies for individuals at increased caries risk.4 Calcium phosphate technologies, a relatively recent innovation, have shown promise in their ability to boost remineralization.1
THE ROLE OF CALCIUM PHOSPHATE TECHNOLOGIES
Topical fluoride ions, in the presence of calcium and phosphate ions, promote the formation of fluorapatite in tooth enamel through remineralization. In order for the remineralization process to occur, calcium and phosphate ions must be available.
Demineralization of tooth enamel involves the breaking down of mineral ions. Tooth enamel is a crystalline latticework composed of diverse minerals, including a calcium phosphate mineral known as hydroxyapatite (Figure 3). Caries lesions are formed when too many minerals are destroyed in an area of the hydroxyapatite latticework. Fortunately, the crystalline latticework of the tooth enamel can be reinforced and repaired through theprocess of remineralization during the early stages of demineralization.
Calcium phosphate technologies are designed to supply the oral environment with additional calcium and phosphate ions. Individuals with adequate saliva typically have these ions readily available. For those with reduced salivary flow or other health problems that may limit the ability of saliva to act as a buffering system and supply an adequate amount of calcium and phosphate ions, calcium phosphate technologies can be an effective solution. Individuals at increased caries risk can also benefit from their use. Four types of calcium phosphate systems are available: amorphous calcium phosphate (ACP); casein phosphopeptide (CPP)-ACP, or Recaldent®; calcium sodium phosphosilicate (CSP), or NovaMin®; and tricalcium phosphate (TCP).
AMORPHOUS CALCIUM PHOSPHATE

ACP delivers calcium salt and phosphate salt to supply enough calcium and phosphate ions to saturate defects in tooth enamel.5 Once released, the ions react on the enamel in the dentinal tubules to provide a source of calcium and phosphate ions in the saliva that is now available to support the remineralization process.6 ACP is available in dentifrice, fluoride gel and varnish, prophy paste, sealants, whitening and desensitizing products, and cements.4,7
CASEIN PHOSPHOPEPTIDE-AMORPHOUS CALCIUM PHOSPHATE
CPP-ACP is derived from casein, which is part of a protein found in cow’s milk. It binds to fluoride and bacteria in the oral cavity and stabilizes ACP, thereby providing a reservoir of calcium and phosphate ions to boost the remineralization process. Limited research demonstrates that CPP-ACP may interfere with the demineralization process and support the remineralization process by increasing calcium and phosphate ion levels in dental biofilm, supporting remineralization of enamel, slowing the development of coronal caries, and providing some level of protection in an acidic environment. 8–10 CPP-ACP comes in a paste (with or without fluoride) that is applied topically to the teeth once or twice daily, in addition to fluoride varnish and chewing gum.7 Because CPPACP is derived from milk protein, patients who are allergic to dairy should avoid its use.
CALCIUM SODIUM PHOSPHOSILICATE
When exposed to saliva, CSP—a bioactive glass—releases ions of calcium, phosphate, and sodium.11,12 The calcium and phosphate ions are buffered by the sodium ions, which precipitate into hydroxyapatite in order to fill demineralized defects.12,13 CSP is found in toothpastes, a prophy paste, desensitizing agents, and, most recently, in an air polishing powder.7 CSP is most commonly used as a desensitizing ingredient.12,13
TRI-CALCIUM PHOSPHATE
TCP is a consolidation of beta tri-calcium phosphate and sodium lauryl sulfate that creates a “functionalized” calcium and a “free” phosphate, which work with fluoride to support the remineralization of enamel subsurface lesions. A shielding barrier is formed around the calcium, allowing it to exist collectively with fluoride when added to toothpaste.14 TCP is available in a prescription-only dentifrice and fluoride varnish.7
CONCLUSION
The demineralization and remineralization process has a fundamental influence on the overall hardness and strength of tooth enamel. In order to sustain strong, healthy tooth structure, the ratio between the demineralization and remineralization processes must be stable. The gold standard in preventing caries is through the topical and systemic use of fluoride. For patients with compromised salivary flow or individuals at elevated caries risk, calcium phosphate technologies may help push the scale toward remineralization. Additional in vivo research and randomized controlled trials are necessary to fully support the efficacy of these ingredients in the remineralization process, but, when used in conjunction with fluoride therapy, they may offer important benefits to the caries prevention process.
References
- Su N, Marek CL, Ching V, Grushka M. Cariesprevention for patients with dry mouth. J CanDent Assoc. 2011;77:b85.
- Diefenderfer KE, Stahl J. Caries remineralizationtherapy: implications for dental readiness. MilMed. 2008;173(1 Suppl):48–50.
- Centers for Disease Control and Prevention.Untreated Dental Caries (Cavities) in Children Ages2-19, United States. Available at: www.cdc.gov/Features/dsUntreatedCavitiesKids. AccessedSeptember 23, 2013.
- Peters MC, Kanjirath PP, Barros JA. Promoteremineralization. Dimensions of Dental Hygiene.2011;9(10):42–47.
- Zhao J, Liu Y, Zhang H. Amorphous calciumphosphate and its application in dentistry. ChemiCent J. 2011;5:40.
- Chow LC, Takagi S, Vogel GL. Amorphouscalcium phosphate: the contention of bone.J Dent Res. 1998;77:6.
- Shah A. Remineralize with calcium-basedtherapies. Dimensions of Dental Hygiene.2013;11(3):30–35.
- Reynolds EC. Calcium phosphate-basedremineralization systems: scientific evidence?Aust Dent J. 2008;53:268–273.
- Reynolds EC, Cai F, Cochrane, NJ, et al. Fluorideand casein phosphopeptide-amorphous calciumphosphate. J Dent Res. 2008;87:344–348.
- Reynolds EC, Cai F, Shen P, Walker GD.Retention in plaque and remineralization ofenamel lesions by various forms of calcium in amouthrinse or sugar-free chewing gum. J DentRes. 2003;82:206–211.
- Golpayegani M, Sohrabi A, Biria M. Ansari G.Remineralization effect of topical novaminversus sodium fluoride (1.1%) on caries-likelesions in permanent teeth. J Dent (Tehran).2012;9:68–75.
- Andersson OH, Kangasniemi I. Calciumphosphate formation at the surface of bioactiveglass in vitro. J Biomed Mater Res.1991;25:1019–1030.
- Burwell AK, Litkowski LJ, Greenspan DC.Calcium sodium phosphosilicate (Novamin):remineralization potential. Adv Dent Res.2009,21:35–39.
- Karlinsey RL, Mackey AC. Solid-statepreparation and dental application of anorganically modified calcium phosphate.Journal of Materials Science. 2009;44(1):346–349.
From Dimensions of Dental Hygiene. October 2013;11(10):40,42,44.

