Assessment is Key to Implant Success
In providing optimal care, soft tissue, bone, and implant restorative components must be routinely evaluated.
With prevention as the main focus of dental hygiene, tooth loss is to be avoided in the quest for oral health. However, sometimes it is unavoidable. Fortunately in modern dentistry, implant supported restorations are a common and predictable method of tooth replacement and the dental hygienist is an important team member in determining the immediate and future care for these restorations. Accurately assessing the peri-implant tissues for health or disease is integral to effective treatment planning and, in the long run, to the success of the implant.
Osseointegrated implant dentistry can effectively meet the restorative needs of the fully and partially edentulous patient.1 However, both implants and the restorations they support can fail in response to local and systemic etiologic factors.2
Understanding the differences between the soft tissue surrounding an implant and the soft tissue around a tooth is essential to proper assessment. Clinically and histologically, the soft tissue around teeth and implants resemble each other. Both are covered by epithelium and form a sulcus that terminates apically with a junctional epithelium adherent to the tooth and implant, respectively.3 However around the implant, the connective tissue located between the junctional epithelium and the crest of the bone differs in several respects due to the absence of a periodontal ligament and cementum. The connective tissue around the implant is adherent and the collagen fibers run parallel to the titanium surface. In contrast around the tooth, the fibers within the connective tissue insert into the cementum of the root.3 The peri-implant connective tissue contains more collagen and fewer fibro- blasts and blood vessels compared to the gingival tissue surrounding a tooth.
This structural difference in the connective tissue between teeth and implants helps explain differences in the presence of inflammation. The inflammatory lesion around implants compared to the lesion around teeth is larger in size and progresses more apically, frequently extending directly into the bone marrow.4 The arrangement of blood vessels in the peri-implant mucosa vs the gingiva may explain the difference in bone loss patterns. The lesion around implants is typically a circumferential trough. In periodontitis around teeth, the bone may be destroyed only on one surface of the tooth.
Bone loss around implants results from occlusal overload5 or plaque induced inflammation.6 Inflammatory disease in the peri-implant tissue is called peri-implant disease. Peri-implant mucositis applies to inflammation limited to the soft tissues. Peri-implantitis describes the loss of supporting bone secondary to inflammation (Figures 1 and 2).7
|
Abnormal tissue color around an implant supported restoration. Notice ulceration of crevicular lining. See Figure 2. |
|
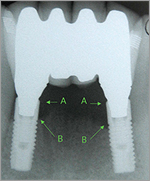
Radiograph reveals peri-implantitis associated with inflammatory changes as seen in Figure 1. Arrow A represents osseous crest at time implant restoration was placed. Arrow B represents osseous crest 15 months later as a result of peri-implant disease. |
|
Implant #4 position appears to have no bone as demonstrated in this panoramic film. |
Bacterial Influences
Many differences exist within the microbial flora of the implant crevice in both health and disease. A healthy population contains predominately gram positive cocci and non-motile rods, whereas a diseased population consists of gram negative anaerobic rods and spirochetes. Furthermore, there are microbial differences between the fully and partially edentulous patient.8 Teeth that may harbor putative periodontal pathogens serve as a reservoir that can ultimately colonize the implants.9 New research documents a more specific trend where these organisms are transmitted from teeth to implants in the same arch.10
The role of bacteria in peri-implant disease impacts significantly on implant dentistry. For example in the partially edentulous patient, pre-existing inflammatory periodontal disease should be controlled before introducing an implant into the oral cavity. The bacterial population should also be controlled daily by the patient and through debridement by the hygienist. The lack of effective plaque control with resultant peri-implantitis affects the rate of bone loss around the implant and the restoration it supports.11
Most crucial to the success of an implant restoration is how effectively the patient maintains the oral environment. This determination needs to be ascertained prior to implant placement. If the patient is not demonstrating effective bacterial control around the natural dentition-particularly with textured or coated surfaces-implants may not be the best treatment option. The periodontal pathogens that affect the natural dentition can be equally damaging to the supporting structures of the implant.
Systemic Influences
Certain systemic influences interfere with the ability to achieve osseointegration and, thus, the prognosis of an implant. Over time, the systemic status of the patient can change, which can influence local etiologic factors, soft tissue, and bone biology. Smoking affects both the oral flora12 and bone metabolism. Certain medications affect the oral flora, the soft tissue, bone metabolism, and parafunctional habits. Common systemic diseases, such as diabetes and osteoporosis, also affect the bone-to-implant contact.13
Occlusion
Occlusal overload can cause loss of integration.14 Occlusal forces can damage the restoration, resulting in excessive wear or fracture of veneering material. These forces can result in the loosening of or fracture of screws that retain restorations or abutments to the underlying implant fixture. Case reports have shown that fracture of the implant can result from excessive occlusal load.15 The loss of integration from occlusal overload is an all or nothing phenomenon. The entire implant becomes fibrous encapsulated and loose all at once. The loss does not occur at the bone crest and work its way down, like a tooth in hyperocclusion.
Assessment Phase
Clinical assessment of the implant patient begins with a medical history review. When considering implant supported restorations, patients’ long-term dental prognosis and potential changes in systemic health (often due to age) must be taken into account.
The intraoral examination must be accompanied by a contemporary series of radiographs. In the partially edentulous patient with full coverage restorations, the radiograph is the only way to determine which restorations are tooth or implant supported. A parallel film is the most sensitive instrument available to execute the clinical assessment and evaluate the crestal bone levels around implants.
Panographic imaging does not usually provide sufficient detail (Figure 3). Panoramic films should only be used when anatomic limitations, such as a shallow vault or floor of the mouth, preclude taking periapical films. The recommended protocol is a base line film taken at the time of insertion of the final prosthesis and then repeated at 6 months, 1 year, and every 2 years hence. This frequency should be adjusted in the face of systemic or clinically observed changes.
Evaluation of the peri-implant mucosa begins by determining whether the tissue is keratinized or nonkeratinized. The color and texture of the mucosa are different when disease is present. In some cases, the lack of keratinized tissue around an implant can limit the patient’s plaque control habits due to increased discomfort. Digital palpation of the tissue in the vicinity of the margin can reveal the presence of bleeding or suppuration. The cornerstone of a periodontal examination around teeth is probing the crevice to measure depth and determine the presence of inflammation manifested by bleeding. However with implants, the depth may not be meaningful as the peri-implant crevice is created surgically and not the result of development. Studies show that bleeding on probing occurs in peri-implant tissues that are histologically devoid of inflammation.16 Radiography, rather than probing, is the most effective discriminator between health and disease around implants (Figure 4).17 The configuration of the restoration can limit access or interfere with parallel placement of the probe. Moreover, threads or textures on the implant surface, as well as the anatomy of the implant and the restoration, can restrict the path of the probe tip (Figure 5).
|
Periapical film reveals more accurate information demonstrating bone is present around implant #4. |
Soft tissue changes around implant restoration. Probing inaccurate measurement due to shape of crown (mesial cantilever) and shape of implant fixture. |
Assessing screw retained prosthesis for any loose parts by putting pressure in the occlusal direction. Abutment screws broken. Notice gap between abutment and implant fixture. |
Mobility of an implant is synonymous with the loss of integration. To check for mobility other than in single unit situations, the restorative superstructure must be removed. This may not need to be done routinely but is indicated if there are soft tissue or radiographic changes suggestive of peri-implant mucositis or peri-implantitis.
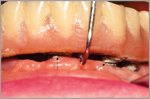
The restoration supported by the implants also requires close scrutiny. The integrity of the veneering material and solder joints needs to be checked. The retention of a fixed prosthesis is determined by placing an instrument, such as a curet, under the restoration while trying to lift it in an occlusal direction (Figure 6). Movement reflects screw looseness or fracture or loss of the integrity of the cement seal. The implications of these findings might imply excessive occlusal forces or inaccurate fit of the superstructure. A restoration that is loose on one or more abutments might stress and damage other implants, abutments, or screws. Furthermore, the resultant movement, particularly if the margin is in the vicinity of the soft tissue, can result in mechanical irritation or increased concentrations of bacteria in the crevice, fostering inflammation. In a screw-retained restoration, integrity of the material used to seal the access hole should also be checked. Wear patterns of the implant-supported restoration or opposing occlusion might indicate the need for occlusal adjustment or an occlusal guard to protect against parafunctional habits. The emergence of implant dentistry as one of the principle means of solving restorative problems means that hygienists will likely encounter patients with implants in day-to-day practice. The hygienist needs to be aware of the host response and the continuously changing factors that can be influential in disturbing the equilibrium defined as a “state of health.”
REFERENCES
- Jemt T, Lekholm U. Oral implant treatment in posterior partially edentulous jaws: a 5-year follow-up report. Int J Oral Maxillofac Implants. 1993;8:635-640.
- Quirynen M, Naert I, van Steenberghe D. Fixture design and overload influence marginal bone loss and fixture success in the Branemark System. Clin Oral Implants Res. 1992;3:104-111.
- Buser D, Weber HP, Donath K, Fiorellini JP, Paquette DW, Williams RC. Soft tissue reactions to non-submerged unloaded titanium implants in beagle dogs. J Periodontol. 1992;63:225-235.
- Lindhe J, Berglundh T, Ericsson I, Liljebberg B, Marinello C. Experimental breakdown of peri-implant and periodontal tissues. A study in the beagle dog. Clin Oral Implants Res. 1992;3:9-16.
- Rosenberg ES, Torosian JP, Slots J. Microbial differences in 2 clinically distinct types of failures of osseointegrated implants. Clin Oral Implants Res. 1991;2:135-144.
- Mombelli A, van Oosten MA, Schurch E Jr, Land NP. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol Immunol. 1987;2:145-151.
- Albrektsson T, Isidor F. Consensus report of Session IV. Lang NP, Karring T, eds. In: Proceedings of the First European Workshop on Periodontology. London: Quintessence; 1994:365-369.
- Apse P, Ellen RP, Overall CM, Zarb GA. Microbiota and crevicular fluid collagenase activity in the osseointegrated dental implant sulcus: a comparison of sites in edentulous and partially edentulous patients. J Periodontal Res. 1989;24:96-105.
- Quirynen M, Listgarten MA. Distribution of bacterial morphotypes around natural teeth and titanium implants ad modum Branemark. Clin Oral Implants Res. 1990;1:8-12.
- Quirynen M, Papaioannou W, van Steenberghe D. Intraoral transmission and the colonization of oral hard surfaces. J Periodontol. 1996;67:986-993.
- Kourtis SG, Sot iriadou S, Voliotis S, Challas A. Private practice results of dental implants. Part I: survival and evaluation of risk factors-Part II: surgical and prosthetic complications. Implant Dent. 2004;13:373-385.
- van Winkelhoff AJ, Bosch-Tijhof CJ, Winkel EG, van der Reijden WA. Smoking affects the subgingival microflora in periodontitis. J Periodontol. 2001;72:666-671.
- Beikler T, Flemmig TF. Implants in the medically compromised patient. Crit Rev Oral Biol Med. 2003;14:305-316.
- Isidor F. Loss of osseointegration caused by occlusal load of oral implants. A clinical and radiographic study in monkeys. Clin Oral Implants Res. 1996;7:143-152.
- Rangert B, Krogh PH, Langer B, Van Roekel N. Bending overload and implant fracture: a retrospective clinical analysis. Int J Oral Maxillofac Implants. 1995;10:326-334.
- Ericsson I, Lindhe J. Probing depth at implants and teeth. An experimental study in the dog. J Clin Periodontol. 1993;20:623-627.
- Weber HP, Crohin CC, Fiorellini JP. A 5-year prospective clinical and radiographic study of non-submerged dental implants. Clin Oral Implants Res. 2000;11:144-153.
From Dimensions of Dental Hygiene. July 2005;3(7):14-16.