Assess Periodontal Risk
Risk assessment analysis, monitoring risk factor modification, and managing risk factors in patients with periodontal diseases, or those at high risk, are essential to successful treatment.
This course was published in the October 2013 issue and expires 10/31/16. The author has no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Describe the risk factors for periodontal diseases.
- Explain the mechanisms that mediate risk factors in periodontal diseases.
- Identify risk factors for periodontal diseases in dental patients.
THE PERIODONTAL PROBLEM
Periodontal diseases, the infection-mediated destruction of tooth-supporting tissues, are some of the most common chronic infections, and the number one cause of tooth loss among adults. The distribution of periodontal disease severity follows a bell curve, with a small segment of the population experiencing no disease or very mild forms; approximately 15% experiencing severe periodontal disease; and a large proportion of the US population experiencing various degrees of periodontal destruction.1 The identification of differences in disease severity led to the concept of differences in disease susceptibility. The study of differences in d isease susceptibility, in turn, led to the identification of risk factors.
A risk factor for periodontal diseases is defined as a characteristic, an aspect of behavior, or an environmental exposure that is associated with the presence of periodontitis. A risk factor might be in the causal chain of disease development, though its presence is associated with disease expression. From the standpoint of risk reduction, risk factors are divided into two categories: modifiable or nonmodifiable. Similar to successful management of chronic multi-factorial diseases, identification and management of risk factors in patients with periodontal diseases are essential for successful disease control. Understanding risk factor modification is essential to good clinical dental practice. The reduction of risk—that is, elimination, modification, or management of the individual risk factors—is critical for successful treatment outcomes and long-term disease control. Risk factors are classified according to their etiology as systemic, environmental, behavioral, or genetic.
SYSTEMIC RISK FACTORS
Diabetes is the most significant systemic risk factor for periodontal diseases. The most common forms of diabetes are type 1 and type 2. Type 1 diabetes (about 5% of diabetes cases) is the result of destruction of pancreatic beta cells and requires the absolute dependence on exogenous insulin to sustain life. The more common form of diabetes—type 2—is chronic and is associated with inflammation and defects in insulin secretion, action, and insulin resistance. Unlike type 1 diabetes, in which patients are exclusively dependent on exogenous insulin, the health of patients with type 2 diabetes is managed mostly with diet modification, physical activity, and oral diabetes medications. The alarming increase in worldwide diabetes cases is due to an increase in type 2 diabetes. Most scientific evidence on the relationship between periodontal diseases and diabetes comes from studies of type 2 diabetes.
Periodontal diseases are more common in people with diabetes. Among young adults, those with diabetes are twice as likely to develop periodontal diseases than those without diabetes. Adults age 45 and older with poorly controlled (A1c >9) diabetes are 2.9 times more likely to have severe periodontitis compared to those without diabetes. The risk increases to 4.6 among current smokers with poorly controlled diabetes.2 Once periodontal diseases are established in a diabetic host, the chronic nature of periodontal infection/inflammation constitutes a risk for difficulty in glucose control and diabetes severity. Thus, the relationship between diabetes and periodontal diseases is two-way: diabetes increases the risk for periodontitis, while periodontal infection and inflammation make diabetes more difficult to control.3
Moderate to severe periodontitis is associated with an increased risk for macroalbuminuria end-stage renal disease, calcification of atherosclerotic plaques, carotid intima-medial thickness, and even cardiorenal mortality.4 There is a direct and dose-dependent relationship between periodontitis severity and diabetes complications in both type 1 type 2 diabetes, with emerging evidence that periodontitis may predispose individuals to the development of diabetes.5–7 New guidelines have been proposed to physicians, dental practitioners, and patients affected by or at risk of diabetes regarding periodontal care and management of oral health.8 These principles state that patients with diabetes should be educated by both medical and dental professionals that their risk of periodontal diseases is high; furthermore, if they are diagnosed with a periodontal disease, their glycemic control may be difficult to manage and poses a great risk for diabetes complications. Periodontal examinations should be performed on all patients with diabetes as part of ongoing management of the disease. Annual periodontal examinations are recommended, even in the absence of periodontitis.8 A relationship between patients’ oral health and medical providers must exist in order to ensure the proper management of diabetes.
OBESITY AND METABOLIC SYNDROME
The most important risk factor for type 2 diabetes is obesity. A systematic review and meta-analysis of the literature concluded that obesity—measured as increased body mass index (BMI)—is associated with increased severity of periodontal diseases.9 Insulin resistance, a condition in which higher levels of insulin are required to maintain normal glucose levels, mediates the increased risk for type 2 diabetes and periodontal diseases in obese individuals.10 Accordingly, waist circumference—a measure of central obesity/abdominal fat that is more closely related to insulin resistance vs whole body BMI—is better associated with periodontal diseases than is a BMI score. The increased risk for periodontal diseases associated with obesity is proportional to the degree of obesity, with the risk increasing for every additional centimeter of waist circumference or unit of BMI score.
Metabolic syndrome also significantly increases the risk of developing cardiovascular disease and diabetes. A combination of factors, including abdominal obesity, insulin resistance, abnormal lipids, and hypertension, contribute to this risk. The association between periodontitis and metabolic syndrome has been reported, though the possible confounding effect of diabetes risk has not been adequately accounted for in most of the published reports. D’Aiuto et al examined data from the National Health and Nutrition Examination Survey III and found an association between metabolic syndrome and severe periodontitis only in individuals older than 45.11
OSTEOPOROSIS AND OSTEOPENIA
Osteopenia is a systemic condition in which bone mineral density (BMD) falls below a predefined level considered adequate for age and gender. Contrarily, osteoporosis is a disease where BMD is below the levels required for mechanical support. Thus, osteoporosis is the presence of bone fracture, or the increased risk of bone fracture. Clear and solid evidence supports the role of osteopenia and osteoporosis as risk factors for tooth loss in both dentate and edentulous individuals.12,13 Krall et al found that for each 1%-per-year decrease in whole body BMD, the risk for tooth loss more than quadrupled in post-menopausal women.13 Women who are at risk of or have osteoporosis are at greater risk of tooth loss as well. Although the evidence supporting osteoporosis and osteopenia as risk factors for periodontal diseases is not as clear as it is for tooth loss, it nevertheless points to an association between osteopenia and osteoporosis and periodontal diseases.14,15 This association is mediated by estrogen, calcium, and vitamin D. Information on skeletal BMD, hormonal, and vitamin supplementation status should be ascertained as part of a complete medical history of postmenopausal female periodontal patients.
ENVIRONMENTAL RISK FACTORS
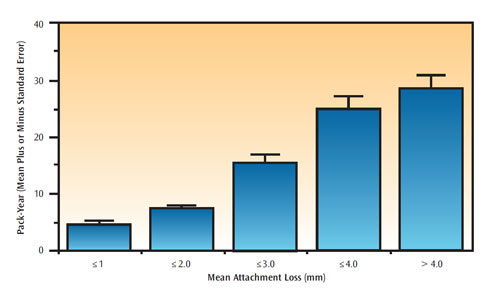
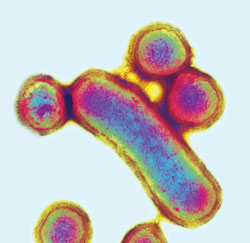
Smoking is the single most important environmental and modifiable risk factor for periodontal diseases. Smokers have, on average, 2.5 times the risk of severe periodontal diseases compared to nonsmokers—independent of age, gender, presence of dental plaque, or diabetes. The odds for periodontal diseases range from 2.0 to 5.0, depending on the amount of smoking (ie, number of cigarettes smoked per day and for how many years).16 Furthermore, the risk is cumulative and dose-dependent, in that the severity of periodontal diseases is positively and directly related to the duration and amount of smoking (Figure 1). When assessing a patient’s smoking history, it is important to determine not only whether the patient smokes, but also the amount of smoking per day and the number of years spent smoking. In general, patients who have smoked 20 pack-years, (ie, smoking one pack per day, every day, for 20 years) exhibit severe periodontal diseases (Figure 1). The negative effect of cigarette smoking is even more pronounced on alveolar bone than soft tissue. Smokers with periodontal diseases are more likely to exhibit severe alveolar bone loss, vertical bony defects, and furcation involvements compared to nonsmokers with periodontal diseases. Cigarette smoking increases the risk for colonization with Porphyromonas gingivalis (Figure 2), Tannerella forsythia, and Aggregatibacter actinomycetemcomitans.17 Scaling and root planing is less effective at eliminating these pathogenic organisms in smokers compared to nonsmokers.18 In assessing smoking history, it is important to distinguish between current and former smoking. A history of smoking, past or present, is associated with presence of periodontal pockets, clinical attachment loss, and alveolar bone loss. Infection with pathogenic organisms and poor outcome to periodontal therapy are associated with current smoking only.
Smoking is the number one modifiable risk factor. Smoking cessation should be encouraged for all current smokers with periodontal diseases. There is evidence that smoking cessation has a positive effect on periodontitis occurrence and periodontal healing.19
ALCOHOL
Alcohol consumption is associated with a moderate risk of periodontal diseases (30%), measured as clinical attachment loss and in gingival recession.20,21 Periodontal disease risk is associated with the amount ofalc ohol consumed, not the type of alcohol consumed. Increased risk of periodontal diseases is seen in individuals who drink more than five alcoholic beverages per week, compared to those who drink less or not at all. The risk increases to 40% for individuals who consume more than 10 alcoholic drinks per week. The increased risk is independent of the behavioral aspect associated with alcohol—such as poor oral hygiene—and likely mediated by the multiple effects alcohol has on susceptibility to infection, inflammation, immune response, etc.20,21 The effect of alcohol on periodontal tissues in alcoholics has not been studied.
DIETARY RISK FACTORS
Analyses of large population-based datasets revealed that reduced calcium intake and low serum calcium levels are associated with increased risk for periodontal diseases.22 Low dietary calcium intake was associated with increased attachment loss in a dose-dependent manner in a sample representative of the US population.23 Low dietary intake of vitamins C and D are also associated with increased risk for periodontal diseases.23,24 Low levels of vitamin D intake result in decreased serum levels of calcium, which in turn stimulates the parathyroid gland to produce parathyroid hormone—causing increased osteoclastogenesis.25 Assessing dietary intake of calcium and vitamins C and D should be part of the periodontal exam.
BEHAVIORAL RISK FACTORS
Psychological stress, distress, and depression have been reported to increase the risk for periodontal diseases.26 A large population-based study reported that individuals with high financial stress exhibited increased severity of periodontal diseases compared to those with lower financial stress.26 Coping behaviors modified the negative effects of stress on the periodontium. That is, individuals with high levels of problem-focused coping (a form of adequate coping) where not at a greater risk for periodontal diseases compared to individuals with high stress and emotion-focused coping (a form of inadequate coping). Thus, adequate coping behaviors may reduce the stress-associated risk for periodontal diseases. Stress, distress, and depression may be associated with periodontal diseases through behavioral and physiologic mechanisms. Behavioral mechanisms include changes in poor oral hygiene, unhealthy diet, and other behaviors not conducive to oral health. Physiologic mechanisms are mediated by cortisol, a hormone secreted during stress that affects inflammations, as well. Assessing stress, distress, depression, and coping behaviors is indicated in periodontal practice.
GENETIC RISK FACTORS
There is evidence suggesting a role for genetic factors in aggressive periodontitis. The genetic marker responsible for the increased risk of aggressive periodontitis includes polymorphisms in the IL-1B gene, which is responsible for up to three-fold increases in IL-1 production.27 Polymorphisms at the IL-1A and IL-1B genes are associated with chronic adult periodontitis only in nonsmokers. In smokers, severe periodontal diseases were not associated with any of the examined candidate polymorphisms. Although genetic factors, such as specific gene polymorphisms, are suspected to have an association with chronic adult periodontitis, there is yet no clear evidence for this in the general population.
BACKGROUND RISK FACTORS
Age and gender are considered background, or nonmodifiable, risk factors. Studies of natural history of periodontal diseases show that severity of periodontal destruction increased with age.28,29 In a large cross-sectional study, clinical attachment loss severity was directly proportional to an increase in age.16 Periodontal diseases are also reported to be more prevalent or more severe in men than in women of comparable ages.16
ASSESSMENT OF RISK FACTORS IN CLINICAL PRACTICE
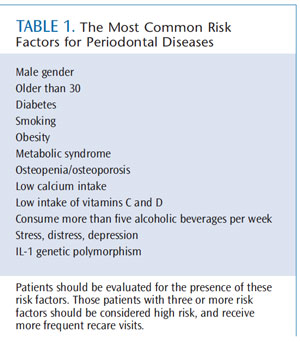
Table 1 lists the most common risk factors for periodontal diseases. Assessing the presence of these factors in individual patients should be a standard component of the initial and follow-up periodontal evaluation. The concept of risk assessment has been incorporated into the American Academy of Periodontology (AAP) guidelines for the management of patients with periodontal diseases, and the web-based AAP patient self-assessment tool for periodontal diseases (www.perio.org). The AAP guidelines describe risk assessment as increasingly important in periodontal treatment planning and should be part of every comprehensive dental and periodontal examination.
RISK FACTOR MODIFICATION IN CLINICAL MANAGEMENT
Management of periodontal diseases that incorporates risk factor modification/reduction is part of contemporary treatment of periodontal diseases. Smoking cessation is the best example of risk factor modification, and supporting patients in quitting tobacco use is an ethical obligation for all dental team members. Dental hygienists in particular should acquire tobacco-use intervention skills. The US Public Health Service’s Clinical Practice Guideline, Treating Tobacco Use and Dependence: 2008 Update, is the gold standard for clinical tobacco intervention services.30 Periodontal treatment in patients with diabetes improves periodontal status and diabetes outcomes measured by reduction in levels of A1c.8 Dental professionals should stress the importance of weight management and promote healthy lifestyles.
Dental hygienists are uniquely positioned to conduct risk assessment analysis, monitor risk factor modification, and help manage risk factors in patients with or at high risk of periodontal diseases.
References
- Dye BA, Tan S, Smith V, et al. Trends in oral health status: United States, 1988-1994 and 1999-2004. Vital Health Stat 11. 2007;248:1–92.
- 2011 National Diabetes Fact Sheet: Diabetes Public Health Resource. United States Centers for Disease Control and Prevention. Available at: www.cdc.gov/diabetes/pubs/estimates11.htm. Accessed September 23, 2013.
- Grossi SG, Genco RJ. Periodontal disease and diabetes mellitus; a two-way relationship. Ann Periodontol. 1998;3:51–61.
- Sareni A, Nelson RG, Tulloch-Reid M, et al. Periodontal disease and mortality in type 2 diabetes. Diabetes Care. 2005;28:27–32.
- Thortensson H, Kuylenstierna J, Hugoson A. Medical status and complications in relation to periodontal disease experience in insulin dependent diabetics. J Clin Periodontol. 1996;23:194–202.
- Shultis WA, Weil EJ, Looker HC, et al. Effect of periodontitis on overt nephropathy and end-stage renal disease in type 2 diabetes. Diabetes Care. 2007;30:306–311.
- Saito T, Shimazaki Y, Kiyohara Y, et al. The severity of periodontal disease is associated with the development of glucose intolerance in non diabetics: the Hiyasama study. J Dent Res. 2004;83:485–490.
- Chapple IL, Genco R, working group 2 of the joint EFP/AAP workshop. Diabetes and periodontal diseases: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Periodontol. 2013;84(Suppl 4):S106–S112.
- Chaffee BW, Weston SJ. Association between chronic periodontal disease and obesity: a systematic review and meta-analysis. J Periodontol. 2010;81:1708–1724.
- Genco RJ, Grossi SG, Ho AW, Nishimura F, Murayama Y. A proposed model linking inflammation to obesity, diabetes and periodontal infections. J Periodontol. 2005;76(Suppl 11): 2075–2084.
- D’Aiuto F, Sabbah W, Netuveli G, Donos N, et al. Association of the metabolic syndrome with severe periodontitis in a large US population-based survey. J Clin Endocrinol Metab. 2008;93:3989–3994.
- Krall EA, Dawson-Hughes B, Papas A, Garcia RI. Tooth loss and skeletal bone density in healthy postmenopausal women. Osteoporos Int. 1994;4:104–109.
- Krall EA, Garcia RI, Dawson-Hughes B. Increased risk for tooth loss is related to bone loss at the whole body, hip and spine. Calcif Tissue Int. 1996;59:433–437.
- Tezal M, Wactawski-Wende J, Grossi SG, Ho AW, Dunford R, Genco RJ. The relationship between bone mineral density and periodontitis in postmenopausal women. J Periodontol. 2000;71:1492–1498.
- Wactawski-Wende J, Hausmann E, Hovey K, Trevisan M, Grossi SG, Genco RJ. The association between osteoporosis and alveolar crestal height in postmenopausal women. J Periodontol. 2005;76(Suppl 11):2116–2124.
- Grossi SG, Zambon JJ, Ho AW, Koch, et al. Assessment of risk for periodontal disease. I. Risk indicators for attachment loss. J Periodontol. 1994;65:260–267.
- Zambon JJ, Grossi SG, Machtei EE, Ho AW, Dunford R, Genco RJ. Cigarette smoking increases the risk for subgingival infection with periodontal pathogens. J Periodontol. 1996;67:1050–1054.
- Grossi SG, Skrepcinski FB, DeCaro T, Zambon JJ, Cummins D, Genco RJ. Response to periodontal therapy in diabetics and smokers. J Periodontol. 1996;67(Suppl 10):1094–1102.
- Grossi SG, Zambon JJ, Machtei EE, et al. Effect of smoking and smoking cessation on healing after mechanical periodontal therapy. J Am Dent Assoc. 1997;128:599–607.
- Tezal M, Grossi SG, Ho AW, Genco RJ. The effect of alcohol consumption on periodontal disease. J Periodontol. 2001;72:183–189.
- Tezal M, Grossi SG, Ho AW, Genco RJ. Alcohol consumption and periodontal disease: The Third National Health and Nutrition Examination Survey. J Clin Periodontol. 2004;31:484–488.
- Nishida M, Grossi SG, Dunford RG, Ho AW, Trevisan M, Genco RJ. Calcium and the risk for periodontal disease. J Periodontol. 2000;71:1057–1066.
- Nishida M, Grossi SG, Dunford RG, Ho AW, Trevisan M, Genco RJ. Dietary vitamin C and the risk for periodontal disease. J Periodontol. 2000;71:1215–1223.
- Hildebolt CF. Effect of vitamin D and calcium on periodontitis. J Periodontol. 2005;76:1576–1587.
- Millen AE, Hovey KM, Lamonte MJ. Plasma 25-hydroxyvitamin D concentrations and periodontal disease in postmenopausal women. J Periodontol. 2013;84:1243–1256.
- Genco RJ, Ho AW, Grossi SG, Dunford RG, Tedesco LA. Relationship of stress, distress and inadequate coping behaviors to periodontal disease. J Periodontol. 1999;70:711–723.
- Kornman KS, Crane A, Wang HY, et al. The interleukin-1 genotype as a severity factor in adult periodontal disease. J Clin Periodontol.1997;24:72–77.
- Ismail AI, Morrison E, Burt BA, et al. Natural history of periodontal disease in adults: findings from the Tecumseh Periodontal Disease Study, 1959-1987. J Dent Res. 1990;69:430–435.
- Neely A, Holford TR, Loe H, Anerud A, Boysen H. The natural history of periodontal disease in man. Risk factors for progression of attachment loss in individuals receiving no oral health care. J Periodontol. 2001;72:1006–1015.
- Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 Update. A U.S. Public Health Service report. Am J Prev Med. 2008;35:158–176.
From Dimensions of Dental Hygiene. October 2013;11(10):58–62.



