
A Whole New World
Guided tissue regeneration offers new treatment possibilities in the field of periodontal therapeutics.
This course was published in the February 2015 issue and expires February 2018. The author has no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Discuss the evolution of guided tissue regeneration.
- Identify the factors that determine the predictability of periodontal regenerative procedures.
- Explain the potential and limitations of guided tissue regeneration.
- Note the role of dental hygienists in periodontal regenerative therapies.
INTRODUCTION
Many studies have been conducted on tissue engineering, which provides a new era of treatment for periodontal defects. In June 2014, Colgate supported the American Academy of Periodontology (AAP) workshop “Enhancing Periodontal Health Through Regenerative Approaches,” held in Chicago. At the meeting, more than 50 experts reviewed the evidence surrounding periodontal regenerative medicine in order to shed light on how to achieve the best treatment outcomes. “A Whole New World,” the first installment of a 2015 series on periodontology, provides a timely review of the existing literature on guided tissue regeneration, evaluates clinical applications, and identifies benefits and limitations of this therapy by explaining the rationale for clinical decision making. The Colgate-Palmolive Company is delighted to have provided an unrestricted educational grant to support this article and the entire educational series created in collaboration with the AAP.
—Matilde Hernandez, DDS, MS, MBA
Dental Science Liaison
Colgate Oral Pharmaceuticals
FROM THE AMERICAN ACADEMY OF PERIODONTOLOGY
As dental professionals, we know that preserving a patient’s natural dentition should be fundamental to any periodontal disease treatment plan, but sometimes patients present with complex cases that may seem hopeless. This is why the area of periodontal regeneration is so exciting. The ability to regenerate bone and tissue lost to periodontitis is revolutionizing how we provide care to our patients. As periodontal regeneration is an area of priority in periodontology, the American Academy of Periodontology (AAP) held a state-of-the-science workshop, “Enhancing Periodontal Health through Regenerative Approaches,” in June 2014. More than 50 experts came together to summarize existing literature, evaluate clinical applications of the science, and identify opportunities for future research. The proceedings from the workshop will be published concurrently in the Journal of Periodontology and Clinical Advances in Periodontics. Keeping dental hygienists informed of periodontal innovations is essential to the AAP’s commitment to collaborative care. I am excited that the AAP is continuing to collaborate with Dimensions of Dental Hygiene and the Colgate-Palmolive Company to offer this educational series. In the article “A Whole New World,” AAP member Thiago Morelli, DDS, MS, provides a foundation for how to select cases that may benefit from guided tissue regeneration and discusses how this “new world” of periodontics offers additional treatment possibilities.
—Joan Otomo-Corgel, DDS, MPH,
President, American Academy of Periodontology
Assistant Clinical Professor, University of California, Los Angeles School of Dentistry
 Disease control is one of the key components of therapeutic management in the field of medical science. Another critical but challenging aspect is regeneration of the affected structures. The more complex the involved tissue or organ, the more difficult the task of reconstitution. Periodontal regenerative therapies are designed to improve the short- and long-term clinical outcomes of periodontally compromised teeth with deep pockets and limited periodontal support. The treatment of teeth with deep pockets associated with deep intrabony defects is clinically challenging. Most authors have classified their prognosis as either questionable or hopeless. If the outcome of these teeth could be moved to fair or favorable, the probability that they could be maintained would significantly improve. The possibility of increasing periodontal support would also help patients improve the comfort and function of their dentition.
Disease control is one of the key components of therapeutic management in the field of medical science. Another critical but challenging aspect is regeneration of the affected structures. The more complex the involved tissue or organ, the more difficult the task of reconstitution. Periodontal regenerative therapies are designed to improve the short- and long-term clinical outcomes of periodontally compromised teeth with deep pockets and limited periodontal support. The treatment of teeth with deep pockets associated with deep intrabony defects is clinically challenging. Most authors have classified their prognosis as either questionable or hopeless. If the outcome of these teeth could be moved to fair or favorable, the probability that they could be maintained would significantly improve. The possibility of increasing periodontal support would also help patients improve the comfort and function of their dentition.
The goals of periodontal regeneration are to obtain new periodontal attachment and bone formation, reduce pocket depth, and minimize gingival recession. The introduction of guided tissue regeneration, which uses tissue-exclusive materials to manipulate cellular kinetics in the healing of periodontal wounds, has offered new treatment possibilities.
BIOLOGICAL FRAMEWORK
New attachment and regeneration have been reported following periodontal surgery in animal and human periodontia, but the results have not always been consistent.1–3 While the regeneration of the epithelial attachment was predictable, the establishment of new cementum and periodontal ligament (PDL) varied greatly in different studies. This may be due to the fast repopulation of the root surface by epithelial cells, hindering the formation of new attachment.4 Research demonstrated the negative influence of epithelium on periodontal regeneration through the achievement of better outcomes when this tissue was excluded.4
The study that pioneered guided tissue regeneration, verifying the regenerative potential of PDL cells, was conducted on three monkeys.5 Fenestration defects were introduced in healthy periodontia and a membrane filter was used to cover them underneath the repositioned mucoperiosteal flaps. When reviewed 6 months later, the histological sections revealed the formation of new attachment that extended to a significant part of the root. Signs of resorption and ankylosis were not noted in the cases where the filter remained intact.5 The preclinical investigation was followed by a human study involving one hopeless mandibular incisor, on which guided tissue regeneration was attempted prior to extraction. The histological analysis of the block section demonstrated approximately 5 mm of new attachment coronal to the crest, confirming the feasibility of the method.6 A further experimental design that used test and control teeth in monkeys showed roots that received membrane therapy exhibited greater new connective tissue attachment and more bone regrowth than the controls.7 Later, Gottlow et al8 published data on 12 clinical cases treated with the same principle—using nonresorbable expanded polytetrafluoroethylene membranes—that showed favorable outcomes.
CLINICAL DETERMINANTS OF SUCCESS
The factors that determine the predictability of periodontal regenerative procedures were elucidated through multivariate analytical models. For this task, a landmark study that included 40 intrabony defects was conducted, and different aspects of therapy were evaluated.9–12 This study set the starting point for future research, and classified the relevant factors into three categories: patient-associated, site-associated, and technique-associated.
Evidence suggests that the clinical outcomes of regenerative therapy are related to how well the periodontitis is controlled. The persistence of poor plaque control, high levels of bleeding on probing,13–16 and elevated bacterial loads of specific microbial pathogens (or complexes of pathogens) have all been associated with poor clinical outcomes.17,18 The level of self-performed plaque control has a large dose-dependent effect on the outcome of periodontal regeneration. Better clinical attachment level gains have been observed in patients with optimal levels of plaque control compared to patients with poor oral hygiene.13,14,19
Smoking also is an independent variable that can negatively affect the healing after guided tissue regeneration procedures. In a retrospective study, Tonetti et al19 evaluated the gained attachment in two groups of patients: those who smoked 10 cigarettes or more per day and nonsmokers. The nonsmokers had better oral hygiene than the smokers, but when the researchers adjusted for this factor, smoking still resulted in significantly less attachment gain after 1 year.19 Smoking also negatively impacted the long-term stability of the gained results and was associated with attachment and tooth loss.20 It is reasonable to assume that any condition or characteristic that modifies the innate wound healing mechanisms, such as diabetes or genetic predispositions, may also compromise the regenerative results.21
The anatomy of the periodontal defect dictates its natural healing potential. Parameters, such as the number of defects, the type of walls in the defect, and its depth and width, are clinically significant. Prichard22 stated that the three-wall, intrabony pocket exerts the greatest potential for regeneration. A clot-supportive configuration and an increased number of surfaces for cell influx could offer plausible explanations for this effect. Cortellini et al10 found that the three walls of the treated defect exhibited the greatest bone fill when compared to two- and one-wall defects (95% vs 82% vs 39%, respectively). A different reading by Tonetti et al,12 however, showed that the number of remaining bony walls might not be the critical factor. These authors found that the depth of the intrabony defect was a key healing determinant.12 It seems that shallow and deep defects exhibit similar potential for regeneration, as expressed by the percentage of clinical attachment level gain, but deeper sites exhibit greater improvements in terms of linear tissue gain.23
The width of the defect, as determined by the radiographic angle, may be another predictor of success. Steffensen and Webert24 showed that defects with an angle less than 45° demonstrated bone gain 15 months to 18 months after surgical therapy, while wider defects were associated with bone loss. Cortellini and Tonetti,25 based on 242 treated intrabony defects, reported better regenerative outcomes with a radiographic defect angle of less than 25° (more attachment gain 1.5 mm on average) than defects greater than 37°. Other studies also have shown that narrower defect angles (<37° or less) and deeper defect depths (?3 mm) are more favorable in terms of regenerative results,26,27 validating the concept that narrower and deeper defects are preferable.
The predictability of guided tissue regeneration in furcation defects is controversial. Studies have shown that Class III28 and mesial/distal maxillary Class II furcations29 constitute clinical challenges for regeneration. The management of mandibular Class II furcations with regenerative therapy appears to be a more realistic approach, judging by the results of different studies.30,31 Some of the above investigations suggested that certain factors could predict the healing outcome. Bowers et al30 concluded that increased distance between the roof of the furcation and the crest and the roof of the furcation and the base of defect increased the depth of horizontal defect. They also found that divergent roots at the crest reduced the possibility for complete closure. Tsao et al31 found that an initial vertical defect less than or equal to 4 mm and the use of a bone graft significantly improved the clinical results. From a radiographic point of view, a long root trunk with a wide furcation entrance and mesio-distal alveolar crest levels apical to the fornix appear to negatively affect outcomes in Class II buccal and lingual furcations.32
The endodontic status of the tooth is another factor that could potentially influence the results of regenerative therapy. A study compared the obtained regenerative gains between vital teeth and teeth that had received successful endodontic therapy. The authors found comparable, favorable results for both groups, which remained stable during the first year post-treatment.33
On the other hand, a mobile tooth may create an unstable wound environment, which, in turn, could compromise the final healing. Conflicting results have been reported regarding the effect of initial tooth mobility on periodontal regeneration. Cortellini et al23 analyzed the data of a multicenter study with a regression model and found that increased mobility led to less attachment gain after 1 year. On the contrary, Trejo and Weltman34 reported that teeth with initial mobility of Miller Class I or II could be successfully treated similarly to teeth without mobility. Clearly, differences in the mobility assessment methods and the applied statistical models may partially explain the disparity in the results of these studies. Nevertheless, teeth in need of occlusal adjustment should be treated prior to any regenerative intervention to minimize the effect of occlusal trauma.
CLINICAL POTENTIAL AND LIMITATIONS
The treatment of intrabony defects is characterized by great variability in terms of the attained results. Aichelmann-Reidy and Reynolds35 analyzed the consistency of results following open-flap debridement, bone replacement grafts, and guided tissue regeneration in this type of defect and found significant variability in outcomes. This is not surprising considering the number of variables that determine the final therapeutic outcome. The authors found that guided tissue regeneration and bone grafts exhibited more consistent success than open flap debridement in increasing clinical attachment levels, as well as in decreasing probing depths.35
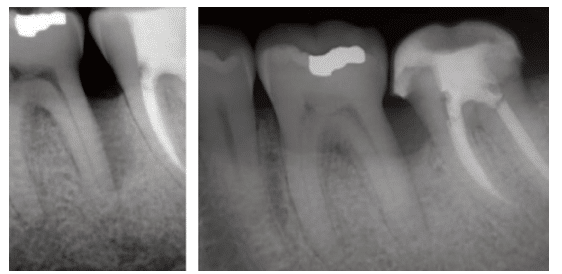
The advantages of guided tissue regeneration in improving clinical attachment levels and increasing bone fill were identified by another systematic review.36 A Cochrane systematic review found also that guided tissue regeneration offers superior outcomes to conventional flap surgery in terms of probing depth reduction, increasing clinical attachment levels, hard tissue probing at surgical re-entry, and decreasing gingival recession.37 They acknowledged, however, there was great variability in the analyzed results. Figure 1 demonstrates the radiographic healing of an intrabony defect treated with guided tissue regeneration. A 6-month radiographic evaluation showed significant bone fill and reduction of the intrabony defect.
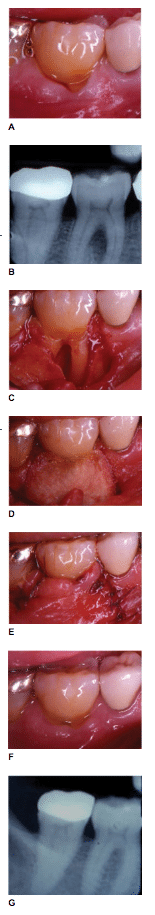
Significant variability is also found in the regenerative outcomes of furcation defects. Jepsen et al39 reported significantly better results with the use of guided tissue regeneration therapy when mandibular and maxillary Class II furcation defects were treated. The authors found decreased horizontal open and closed probing depths, as well as increased horizontal and vertical closed clinical attachment levels. Figure 2 demonstrates the clinical and radiographic healing of a Class II furcation defect treated with guided tissue regeneration.
Another study evaluated the degree of improvement that can be achieved in Class II furcation defects. Evans et al40 reviewed clinical studies with different designs and found that complete furcation closure was accomplished only 20% of the time. Moreover, transformation of Class II furcations to Class I was noted in 33% of the cases, and an overall improvement was reported in about 50% of the treated furcas. Based on this study, the most effective therapy was the combination of membrane and bone graft.40
BIOLOGIC MATERIALS
In the early 2000s, purified or molecular cloned biologic factors were introduced to treat periodontal defects. Enamel matrix derivative—a partially purified material from the developing purified tooth bud that is applied to periodontal lesions to stimulate cementogenesis and periodontal regeneration—was one of the first of these materials.41,42 Platelet-derived growth factor-BB, a recombinant molecule, demonstrated significant efficacy on periodontal regeneration when compared to bone substitute graft material alone.43,44 Other recombinant molecules also demonstrated periodontal repair or regeneration in humans, including fibroblast growth factor-2,45 bone morphogenetic protein-2,46 and teriparatide.47 Biologic materials improve tissue regeneration by enriching the natural biomolecules that may be deficient in chronic periodontal wounds.
THE ROLE OF DENTAL HYGIENISTS
With the development of new materials and surgical techniques, periodontal regeneration has become a predictable and affordable periodontal therapy. Case selection, proper diagnosis, patient education, and follow-up care, however, greatly impact treatment outcomes. Dental hygienists play an important role in all of these areas. The first step in periodontal regeneration is to identify a periodontal defect that may be helped by the technique. Dental hygienists should be familiar with radiographic characteristics of periodontal defects, especially vertical bone defects, which can be treated with regenerative therapies. The characteristics of the defect and the tooth should be considered, such as the depth of the vertical defect (deeper the defect, greater the chance for regeneration), the width of the defect (narrower the defect, better the prognosis), and the number of bone walls circumscribing the defect (the more bone walls, the more predictable the regeneration).
A comprehensive periodontal examination should be completed annually during periodontal maintenance or prophylaxis appointments. During the examination, pocket depth, clinical attachment levels, furcation involvement, tooth mobility, occlusion, and soft tissue characteristics should be evaluated and compared to baseline to assess any changes. Sites with vertical bone loss often present with deeper pocket depths. Periodontal defects are typically more prevalent in the posterior sites48 and sites with wider interdental spaces.49 In addition, intrabony bone defects are more prevalent in the mandibular arch and at the mesial sites.50 Vertical bone defects, however, also can be associated with anterior teeth. A combination of both clinical and radiographic evaluation is essentials for proper periodontal diagnosis.51
Following regenerative therapy, post-surgical care and periodontal maintenance are essential for proper healing. Caution must be used when managing the post-surgical site, as the regenerated tissue can be easily traumatized by instrumentation and probing. Re-evaluation of the surgical site must be completed 6 months after the regenerative therapy. In addition, a consistent 3-month periodontal maintenance interval must be maintained.52
Dental hygienists play an essential role in caring for patients’ periodontal health. Educating patients about their periodontal condition is vital for the success of all periodontal therapy and their role in apprising the dentist on patients’ clinical and radiographic status supports timely diagnosis of periodontal problems.
CONCLUSION
Guided tissue regeneration has revolutionized the treatment of periodontal diseases. The concept has been gradually validated with different levels of evidence, and is now part of the periodontal armamentarium. The results reached in certain cases are impressive and can change the prognosis of questionable teeth. The key to success, however, remains case selection. Clinicians need to weigh all of the factors that may influence outcomes and consider the risk-benefit ratio. The progress made in the science of biomaterials has provided optimized barrier designs, bone grafts, and biologic agents. It is now in the hands of clinicians to plan and execute the therapy in order to achieve the most effective and consistent results possible.
ACKNOWLEDGEMENT
The author would like to thank the American Academy of Periodontology Foundation for supporting his academic career.
REFERENCES
- Listgarten MA, Rosenberg MM. Histological study of repair following new attachment procedures in human periodontal lesions. J Periodontol. 1979;50:333–344.
- Caton J, Nyman S. Histometric evaluation of periodontal surgery. I. The modified Widman flap procedure. J Clin Periodontol. 1980;7:212–223.
- Melcher AH. Repair of wounds in the periodontium of the rat. Influence of periodontal ligament on osteogenesis. Arch Oral Biol. 1970;15:1183–1204.
- Caton J, Zander HA. Osseous repair of an infrabony pocket without new attachment of connective tissue. J Clin Periodontol. 1976;3:54–58.
- Nyman S, Gottlow J, Karring T, Lindhe J. The regenerative potential of the periodontal ligament. An experimental study in the monkey. J Clin Periodontol. 1982;9:257–265.
- Nyman S, Lindhe J, Karring T, Rylander H. New attachment following surgical treatment of human periodontal disease. J Clin Periodontol. 1982;9:290–296.
- Gottlow J, Nyman S, Karring T, Lindhe J. New attachment formation as the result of controlled tissue regeneration. J Clin Periodontol. 1984;11:494–503.
- Gottlow J, Nyman S, Lindhe J, Karring T, Wennstrom J. New attachment formation in the human periodontium by guided tissue regeneration. Case reports. J Clin Periodontol. 1986;13:604–616.
- Cortellini P, Pini Prato G, Tonetti MS. Periodontal regeneration of human infrabony defects. I. Clinical measures. J Periodontol. 1993;64:254–260.
- Cortellini P, Pini Prato G, Tonetti MS. Periodontal regeneration of human infrabony defects. II. Re-entry procedures and bone measures. J Periodontol. 1993;64:261–268.
- Tonetti MS, Pini Prato G, Williams RC, Cortellini P. Periodontal regeneration of human infrabony defects. III. Diagnostic strategies to detect bone gain. J Periodontol. 1993;64:269–277.
- Tonetti MS, Pini-Prato G, Cortellini P. Periodontal regeneration of human intrabony defects. IV. Determinants of healing response. J Periodontol. 1993;64:934–940.
- Cortellini P, Pini Prato G, Tonetti MS. Interproximal free gingival grafts after membrane removal in guided tissue regeneration treatment of intrabony defects. A randomized controlled clinical trial. J Periodontol. 1995;66:488–493.
- Cortellini P, Pini Prato G, Tonetti MS. Periodontal regeneration of human intrabony defects with titanium reinforced membranes. A controlled clinical trial. J Periodontol. 1995;66:797–803.
- Mellonig JT. Human histologic evaluation of a bovine-derived bone xenograft in the treatment of periodontal osseous defects. Int J Periodontics Restorative Dent. 2000;20:19–29.
- Tonetti MS, Prato GP, Cortellini P. Factors affecting the healing response of intrabony defects following guided tissue regeneration and access flap surgery. J Clin Periodontol. 1996;23:548–556.
- Ehmke B, Rudiger SG, Hommens A, Karch H, Flemmig TF. Guided tissue regeneration using a polylactic acid barrier. J Clin Periodontol. 2003;30:368–374.
- Heitz-Mayfield L, Tonetti MS, Cortellini P, Lang NP, European Research Group on Periodontology. Microbial colonization patterns predict the outcomes of surgical treatment of intrabony defects. J Clin Periodontol. 2006;33:62–68.
- Tonetti MS, Pini-Prato G, Cortellini P. Effect of cigarette smoking on periodontal healing following GTR in infrabony defects. A preliminary retrospective study. J Clin Periodontol. 1995;22:229–234.
- Cortellini P, Paolo G, Prato P, Tonetti MS. Long-term stability of clinical attachment following guided tissue regeneration and conventional therapy. J Clin Periodontol. 1996;23:106–111.
- Kornman KS, Robertson PB. Fundamental principles affecting the outcomes of therapy for osseous lesions. Periodontol 2000. 2000;22:22–43.
- Prichard JF. The etiology, diagnosis and treatment of the intrabony defect. J Periodontol. 1967;38:455–465.
- Cortellini P, Carnevale G, Sanz M, Tonetti MS. Treatment of deep and shallow intrabony defects. A multicenter randomized controlled clinical trial. J Clin Periodontol. 1998;25:981–987.
- Steffensen B, Webert HP. Relationship between the radiographic periodontal defect angle and healing after treatment. J Periodontol. 1989;60:248–254.
- Cortellini P, Tonetti MS. Focus on intrabony defects: guided tissue regeneration. Periodontology 2000. 2000;22:104–132.
- Klein F, Kim TS, Hassfeld S, et al. Radiographic defect depth and width for prognosis and description of periodontal healing of infrabony defects. J Periodontol. 2001;72:1639–1646.
- Eickholz P, Horr T, Klein F, Hassfeld S, Kim TS. Radiographic parameters for prognosis of periodontal healing of infrabony defects: two different definitions of defect depth. J Periodontol. 2004;75:399–407.
- Pontoriero R, Lindhe J. Guided tissue regeneration in the treatment of degree III furcation defects in maxillary molars. J Clin Periodontol. 1995;22:810–812.
- Pontoriero R, Lindhe J. Guided tissue regeneration in the treatment of degree II furcations in maxillary molars. J Clin Periodontol. 1995;22:756–763.
- Bowers GM, Schallhorn RG, McClain PK, Morrison GM, Morgan R, Reynolds MA. Factors influencing the outcome of regenerative therapy in mandibular Class II furcations: Part I. J Periodontol. 2003;74:1255–1268.
- Tsao YP, Neiva R, Al-Shammari K, Oh TJ, Wang HL. Factors influencing treatment outcomes in mandibular Class II furcation defects. J Periodontol. 2006;77:641–646.
- Horwitz J, Machtei EE, Reitmeir P, Holle R, Kim TS, Eickholz P. Radiographic parameters as prognostic indicators for healing of class II furcation defects. J Clin Periodontol. 2004;31:105–111.
- Cortellini P, Tonetti MS. Evaluation of the effect of tooth vitality on regenerative outcomes in infrabony defects. J Clin Periodontol. 2001;28:672–679.
- Trejo PM, Weltman RL. Favorable periodontal regenerative outcomes from teeth with presurgical mobility: a retrospective study. J Periodontol. 2004;75:1532–1538.
- Aichelmann-Reidy ME, Reynolds MA. Predictability of clinical outcomes following regenerative therapy in intrabony defects. J Periodontol. 2008;79:387–393.
- Laurell L, Gottlow J, Zybutz M, Persson R. Treatment of intrabony defects by different surgical procedures. A literature review. J Periodontol. 1998;69:303–313.
- Needleman I, Tucker R, Giedrys-Leeper E, Worthington H. Guided tissue regeneration for periodontal intrabony defects—a Cochrane Systematic Review. Periodontol 2000. 2005;37:106–123.
- Reynolds MA, Aichelmann-Reidy ME, Branch-Mays GL, Gunsolley JC. The efficacy of bone replacement grafts in the treatment of periodontal osseous defects. A systematic review. Ann Periodontol. 2003;8:227–265.
- Jepsen S, Eberhard J, Herrera D, Needleman I. A systematic review of guided tissue regeneration for periodontal furcation defects. What is the effect of guided tissue regeneration compared with surgical debridement in the treatment of furcation defects? J Clin Periodontol. 2002;29(Suppl 3):103–116.
- Evans GH, Yukna RA, Gardiner DL, Cambre KM. Frequency of furcation closure with regenerative periodontal therapy. J West Soc Periodontol Periodontal Abstr. 1996;44:101–109.
- Dori F, Arweiler NB, Szanto E, Agics A, Gera I, Sculean A. Ten-year results following treatment of intrabony defects with an enamel matrix protein derivative combined with either a natural bone mineral or a beta-tricalcium phosphate. J Periodontol. 2013;84:749–757.
- Koop R, Merheb J, Quirynen M. Periodontal regeneration with enamel matrix derivative in reconstructive periodontal therapy: a systematic review. J Periodontol. 2012;83:707–720.
- Nevins M, Giannobile WV, McGuire MK, et al. Platelet-derived growth factor stimulates bone fill and rate of attachment level gain: results of a large multicenter randomized controlled trial. J Periodontol. 2005;76:2205–2215.
- Thakare K, Deo V. Randomized controlled clinical study of rhPDGF-BB + beta-TCP versus HA + beta-TCP for the treatment of infrabony periodontal defects: clinical and radiographic results. Int J Periodontics Restorative Dent. 2012;32:689–696.
- Kitamura M, Akamatsu M, Machigashira M, et al. FGF-2 stimulates periodontal regeneration: results of a multi-center randomized clinical trial. J Dent Res. 2011;90:35–40.
- Jung RE, Windisch SI, Eggenschwiler AM, Thoma DS, Weber FE, Hammerle CH. A randomized-controlled clinical trial evaluating clinical and radiological outcomes after 3 and 5 years of dental implants placed in bone regenerated by means of GBR techniques with or without the addition of BMP-2. Clin Oral Implants Res. 2009;20:660–666.
- Bashutski JD, Eber RM, Kinney JS, et al. Teriparatide and osseous regeneration in the oral cavity. N Engl J Med. 2010;363:2396–2405.
- Vrotsos JA, Parashis AO, Theofanatos GD, Smulow JB. Prevalence and distribution of bone defects in moderate and advanced adult periodontitis. J Clin Periodontol. 1999;26:44–48.
- Tal H. Relationship between the interproximal distance of roots and the prevalence of intrabony pockets. J Periodontol. 1984;55:604–607.
- Kim CK, Choi SH, Kim TS, Kaltschmitt J, Eickholz P. The infrabony defect and its determinants. J Periodontol Res. 2006;41:498– 502.
- Schallhorn RA, McClain PK. Periodontal regeneration: management of periodontal osseous defects by the periodontist-dental hygienist team. J Evid Based Dent Pract. 2014;(Suppl14):42–52.
- Ramfjord SP, Morrison EC, Burgett FG, et al. Oral hygiene and maintenance of periodontal support. J Periodontol. 1982;53:26–30.
From Dimensions of Dental Hygiene. February 2015;13(2):63–68.



