 UNDETERMINED/ ISTOCK / GETTY IMAGES PLUS
UNDETERMINED/ ISTOCK / GETTY IMAGES PLUS
A Minimally Invasive Approach to Caries
Prevention is the key to reducing the burden of tooth decay, and a variety of medicaments are available to support this effort.
Dental caries is a prevalent, chronic, and infectious oral disease that continues to plague both adults and children.1 Worldwide, approximately 2.3 billion people have experienced caries in their permanent teeth, and 530 million children are diagnosed with caries in their primary teeth.2 Caries can affect individuals throughout their lifetimes. In its advanced form, caries can cause pain, nutritional deficiency, and poor growth and development outcomes—leading to missed school and workdays and reduced quality of life.1 Further, untreated caries may lead to life-threatening infections.3
Caries disproportionally affects people who have lower socioeconomic status (SES) as well as minorities.4,5 Treating caries presents challenges for patients who do not have access to care or who cannot afford treatment.5 This demonstrates the need for more affordable and less invasive treatments, and the importance of preventive strategies to reduce the caries burden.
Caries is a multifactorial disease based on the interaction between three factors:3,6,7
- Biological (tooth structure and quantity/quality of saliva)
- Substrate (diet high in fermentable carbohydrates and/or sugars and frequency of consumption)
- Bacterial, specifically Streptococcus mutans and Lactobacillus
The bacteria metabolize the fermentable carbohydrates and sugars, producing acid as a byproduct. The acids break down the minerals in the tooth structure, a process known as demineralization.8
The caries process is often described as a balance between preventive factors and pathological factors.7,8 Preventive factors consist of a diet low in fermentable carbohydrates and sugars, adequate saliva, effective oral hygiene, and fluoride exposure. Pathological factors include a diet high in fermentable carbohydrates and sugars, inadequate saliva, and high levels of acid-producing bacteria residing in the plaque/biofilm. There is an ongoing imbalance between the two, which results in a mineral loss (demineralization) or gain (remineralization).8 If the demineralization process outweighs the remineralization process for a prolonged time period, a cavitated lesion results.7,8
Minimally Invasive Dentistry
Over the past few decades, a movement to treat caries conservatively and preserve tooth structure has grown in momentum. This concept is known as minimally invasive dentistry, and it supports the reversal of incipient lesions through remineralization efforts, as well as the prevention of caries entirely.9 It is also in alignment with patients who desire a more holistic approach to dentistry. Fluoride remains the gold standard in caries prevention, however, some patients avoid fluoride exposure and are interested in more natural oral healthcare approaches.7,8,10
The best defense against caries is preventing the lesions from occurring.7,8 Additionally, incipient carious lesions can be halted or reversed with various products and procedures.7 Anticaries products and procedures include the application of fluoride, silver diamine fluoride (SDF), silver nanoparticles, chlorhexidine, dental sealants, xylitol, and calcium phosphate technologies. Arginine is an emerging anticaries product. Table 1 provides an overview of these options. 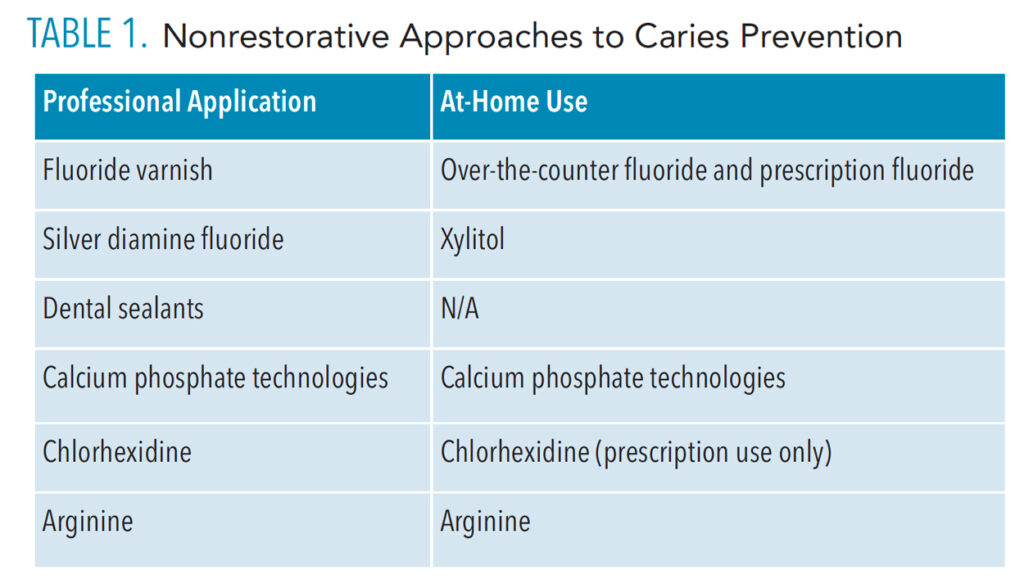
Fluoride
Fluoride is a mineral found in natural sources of water such as groundwater and oceans.10 Fluoride protects the teeth by binding to the tooth structure, replacing the hydroxide anion (oxygen and hydrogen) with fluorohydroxyapatite, thereby strengthening it and making it less susceptible to acid attacks.11 When administered systemically, fluoride is incorporated into the tooth as it forms, while topical application provides protection to erupted teeth.12
Fluoride can be administered topically at higher concentrations through in-office professional application or at-home prescription-strength products, or at lower concentrations, through over-the-counter products. Topical fluoride formulations are available as gels, varnishes, mouthrinses, and dentifrices. Fluoride is also administered systemically through water fluoridation or via supplements, when fluoridated water is not available. In some communities where there is not enough natural fluoride in the drinking water, local officials add additional fluoride to the water supply to help prevent caries for the community at large. The United States Centers for Disease Control and Prevention counts community water fluoridation as one of the top 10 great public health interventions of the 20th century.10 Fluoride is considered a safe and cost-effective method for preventing caries at the community level.10
The benefits of certain types of fluoride are not just limited to caries prevention. For example, fluoride varnish and stannous fluoride can both aid with dentinal hypersensitivity by providing a smear layer. Moreover, stannous fluoride has an antimicrobial effect to help patients who require a product with both anticaries and antigingivitis properties.13
Silver Diamine Fluoride
SDF is an alkaline solution consisting of silver nitrate and fluoride.14 The United States Food and Drug Administration (FDA) has approved it as desensitizing agent. Just as in the case of fluoride varnish, which is also FDA approved as a desensitizing agent, both products exhibit strong anticaries benefits. SDF is also classified as a “breakthrough therapy” by the FDA for caries management.15
SDF prevents demineralization and promotes remineralization of both enamel and dentin. However, whereas fluoride has the ability to prevent caries lesions and remineralize early lesions, SDF takes this a step further by actually halting the caries process. As an anticaries agent, SDF is a 38% formulation consisting of both silver and fluoride. The mechanism of action is similar to other fluoride medicaments, with the added benefit of silver’s antimicrobial properties. The silver ions also serve to block dentinal tubules and prevent contact of the cariogenic bacteria with the tooth surface.14
SDF has been shown to arrest caries at a rate of approximately 70%.14 It is also used as an interim treatment option for patients who require caries arrest, but where a permanent restoration is not possible. SDF contains a fluoride concentration of 44,800 ppm, a much higher amount than fluoride varnish.14 Other advantages are that its application is quick, cost effective, and noninvasive.13 However, SDF is not without drawbacks. The most common downside is the black staining that occurs when the solution comes in contact with the carious lesion. Therefore, esthetic factors must be considered when placing SDF. It also must be provided and monitored by a healthcare professional and is not indicated for home use. Silver nanoparticles also offer anticaries benefits but they are not commercially available at this time.
Chlorhexidine
Chlorhexidine is a broad-spectrum antimicrobial, and is considered one of the strongest chemotherapeutic agents. Chlorhexidine is available as a prescription gel, varnish, mouthrinse, and dentifrice. As an antimicrobial, chlorhexidine alters the ecological system in the oral cavity by suppressing the metabolic function of some bacteria while allowing other resistant bacteria to flourish. As such, safety concerns regarding possible bacterial resistance have arisen.16
Although chlorhexidine is indicated for biofilm control in the treatment of gingivitis, its role as a caries prevention agent has been controversial.9,17 Research indicates chlorhexidine suppresses the proliferation of S. mutans. In addition, rinsing with 0.12% chlorhexidine gluconate in conjunction with a daily dose of high-concentration fluoride toothpaste reduces both coronal and root caries more than when fluoride is used alone.17 Chlorhexidine also carries the risk of temporary brown staining of the teeth. However, use of chlorhexidine gel or varnish minimizes these stains.
Dental Sealants
Dental sealants are a cost-effective means of preventing caries. The thin plastic protective coating provides a physical barrier between tooth surfaces with complex morphology and the acidic by-products of oral bacteria and food debris. Though they have been recognized as a caries-inhibiting procedure for decades, sealants remain underutilized.18,19
Sealants can be distinguished by their method of polymerization (autopolymerized or photopolymerized), filler content (filled or unfilled), and color (clear, tinted, or opaque). The two main types of dental sealants are resin-based and glass ionomer, and each has distinct properties, such as fluoride release and retention rate. According to the literature, resin-based sealants have a better retention rate; however, when comparing their effectiveness in preventing occlusal caries, there is not a significant difference.18
Xylitol
Xylitol is a plant-based, five carbon sugar polyol that has been approved by the FDA as an artificial sweetener.20 As s a sweetener, xylitol is noncariogenic, replacing the highly cariogenic sugar, sucrose.3 In addition, xylitol exhibits anticaries properties. Xylitol inhibits the transfer of glucose to bacteria, specifically S. mutans, that effectively disrupts their energy production process and results in bacterial cell death. When used as a chewing gum, xylitol has the added benefit of increasing salivary flow, adding to the caries protection.21
As a caries preventive agent, xylitol is available as a chewing gum, lozenge, candy, dentifrice, mouthrinse, and wipe. The recommended dosage is 6 g to 10 g divided into four exposures per day. Xylitol can be recommended to patients who avoid fluoride exposure and desire a more natural approach to oral care.
Methods to nourish the dental plaque biofilm with substrates that encourage alkali production may be effective.
Calcium Phosphate Technologies
Amorphous calcium phosphate (ACP) is an essential mineral that provides an artificial source of hydroxyapaptite. It is unstable alone and must be combined with fluoride or another stabilizing agent to offer remineralization properties. Designed to fill microscopic defects on the tooth surface, ACP with fluoride is available in a variety of products including fluoride varnish; prophy paste; and sealant, bonding, and composite materials.22,23
Calcium sodium phosphosilicate (CSP) is a bioactive glass intended to form hydroxyapatite by depositing calcium and phosphate ions on the tooth surface. Its primary indication has been to manage dentinal hypersensitivity, but its remineralizing properties have led to its use in dental products such as prophy pastes and air polishing powders.22,24
Casein phosphopeptides (CPP)-ACP is a remineralization technology that combines calcium and phosphate ions in a soluble agent that binds to saliva, biofilm, soft tissues, enamel, and dentin.25 Research shows that CPP-ACP reduces carious activity on both smooth and fissured surfaces.26 Casein peptides are derived from milk protein and are combined with ACP to form a stabilized solution that supports remineralization and inhibits demineralization by increasing the level of calcium and phosphate present in the oral cavity.25,26 Further, CPP-ACP increases the remineralization effects of fluoride due to the additional presence of calcium and phosphate ions, thus supporting its use as an adjunctive agent to fluoride.26 CPP-ACP is available in a paste, both with and without the addition of fluoride. Other CPP-ACP products include chewing gum and mints.
Tri-calcium phosphate (TCP) is another agent designed to support remineralization. A calcium salt of phosphoric acid, TCP helps to remineralize tooth structure as its application creates a protected barrier, allowing calcium and phosphate to be delivered to the tooth surface. When used in collaboration with fluoride, it is designed to enhance the remineralization of enamel, prolong fluoride release, and offer anti-erosion benefits. TCP is available in a prescription toothpaste and varnish.22,27,28
Arginine
Arginine is an amino acid (2-amino-5-guanidinovaleric acid) that helps the body make proteins. It is produced naturally in the body and is a component of healthy saliva.29 Arginine can also be obtained through dietary supplements or a diet of protein-rich foods such as red meat, poultry, fish, lentils, nuts, soybeans, and dairy products.29–31 Kleinberg and colleagues first discovered arginine in 1979.32 The researchers sought to understand the anticariogenic properties of arginine. Over the years, there has been a considerable amount of research on the topic.
With low pH as a key factor in developing carious lesions, the focus in caries prevention has been to target bacteria that produce acid and their mechanisms of acid production. A recent shift has been to explore an ecologic management of caries prevention by studying microbial metabolic activities that help neutralize biofilm pH.33,34 As opposed to antimicrobials that kill both harmful and beneficial bacteria, methods to nourish the dental plaque biofilm with substrates that encourage alkali production may be an effective alternative.34
Emerging research suggests arginine may be beneficial in preventing caries by making the ecology of the oral environment less hospitable to cariogenic bacteria and more welcoming to healthy bacteria. Individuals with naturally higher salivary levels of arginine have increased caries resistance when compared to individuals with lower arginine levels.34–37
Arginine is metabolized by several arginolytic bacteria—such as S. sanguinis, S. parasanguins, and S. gordonii—via the arginine deiminase pathway to produce ammonia as a byproduct. Ammonia is alkaline, which neutralizes the glycolytic acids in the oral cavity, creating an environment that is hostile to cariogenic bacteria. Ammonia helps to increase the pH in the oral cavity to seven, thereby shifting the oral balance to one favoring health.34 When the pH is maintained at an alkaline level, acidogenic bacteria perish, thereby altering the microbiota in the oral cavity.36,38
Several types of products containing arginine are available including dentifrices, varnishes, and candies. As a caries prevention dentifrice, 1.5% arginine is available with and without fluoride.16,39 However, research demonstrates that toothpaste containing both arginine and fluoride was more effective at caries prevention than arginine alone.16 The addition of 1.5% arginine to a 1,450 ppm fluoridated dentifrice also yielded positive results in caries prevention and the reversal of early lesions.29,40,41
The safety of long-term arginine supplement use is not clear. L-arginine has most often been used by adults in doses that vary from 1.5 g to 24 g by mouth daily, for up to 18 months.36 L-arginine is safe for most people when taken short-term, but can cause stomach pain, bloating, diarrhea, and low blood pressure.42 Not enough is known about its use during pregnancy or breastfeeding. The presence of any of the following is a contraindication for arginine use:
- Guanidinoacetate methyltransferase deficiency (an inherited condition that affects the brain and muscles)
- Recent cardiac event
- Kidney disease
- Upcoming surgery
Prior to taking any medication/supplement, patients should be advised to first consult their physician.42
Arginine can also be used to treat dentinal hypersensitivity by supporting additional distribution of calcium and phosphate in the oral cavity.32 When used as a desensitizer, 8% arginine bicarbonate calcium carbonate is combined with a 1,450 ppm sodium fluoride and can be professionally applied or used at home.32
Lastly, promising research on the benefits of arginine in preventing secondary caries has been conducted.43 Secondary caries is one of the main reasons composite restorations fail. During polymerization, composites shrink, providing a microscopic crevice for the plaque biofilm to invade. The introduction of 7% arginine into dental adhesives to leverage the antimicrobial and anticaries benefits has been evaluated.43 While preliminary results of this in vivo study have been positive, additional research is necessary.
Conclusion
Caries is a disease that requires intervention in its earliest stages. The oral cavity houses various bacteria and to survive, they must withstand constant changes in environmental pH and energy source.44 Patient education, dietary/behavioral changes, and preventive agents are equally important in halting this disease. Clinicians have several agents to choose from to help reduce caries prevalence. In addition to traditional products, arginine is available as an anticaries agent. However, further clinical research is needed to support the claims regarding arginine and promote its widespread clinical acceptance.
Clinicians should familiarize themselves with different available medicaments in an effort to provide the best possible preventive treatment options for their patients.
References
- United States Centers for Disease Control and Prevention. Oral Health Surveillance Report: Trends in Dental Caries and Sealants, Tooth Retention, and Edentulism, United States, 1999–2004 to 2011–2016. Available at: cdc.gov/oralhealth/pdfs_and_other_files/oral-health-surveillance-report-2019-h.pdf. Accessed April 22, 2022.
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858.
- Rathee M, Sapra A. Dental caries. StatPearls. October 6, 2021.
- Fleming E, Afful J. Prevalence of total and untreated dental caries among youth: United States, 2015–2016. NCHS Data Brief. 2018;307:1–8.
- Northridge ME, Kumar A, Kaur R. Disparities in access to oral health care. Annu Rev Public Health. 2020;41:513–535.
- Keyes PH. The infectious and transmissible nature of experimental dental caries. Findings and implications. Arch Oral Biol. 1960;1:304–320.
- Featherstone JDB. The science and practice of caries prevention. J Am Dent Assoc. 2000;131:887–899.
- Featherstone JDB. The continuum of dental caries: evidence for a dynamic disease process. J Dent Res. 2004;83(Spec Iss C):C39–C42.
- Yu OY, Lam WY, Wong AW, Duangthip D, Chu CH. Nonrestorative management of dental caries. Dent J (Basel). 2021;9:121.
- United States Centers for Disease Control and Prevention. Community Water Fluoridation. Available at: cdc.gov/fluoridation/basics/index.htm. Accessed April 22, 2022.
- Buzalaf MAR, Pessan JP, Honório HM, Ten Cate JM. Mechanisms of action of fluoride for caries control. Monogr Oral Sci. 2011;22:97–114.
- American Dental Association. Fluoride: Topical and Systemic Supplements. Available at: ada.org/resources/research/science-and-research-institute/oral-health-topics/fluoride-topical-and-systemic-supplements. Accessed April 22, 2022.
- White DJ. A “return” to stannous fluoride dentifrices. J Clin Dent. 1995;6(Spec No):29–36.
- Sultan A, Akansha J, Gurvinder K. Silver diamine fluoride as a proactive anti-caries tool: a review. International Journal of Oral Health Dentistry. 2019;5(2):63–68.
- United States Food and Drug Administration. Fact Sheet: Breakthrough Therapies. Available at: fda.gov/regulatory-information/food-and-drug-administration-safety-and-innovation-act-fdasia/fact-sheet-breakthrough-therapies#:~:text=FDASIA%20Section%20902%20provides% 20for,threatening%20disease%20or%20condition%20andAccessed April 22, 2022.
- Bijle MN, Ekambaram M, Yung Yiu CK. A scoping review on arginine in caries prevention. J Evid Based Dent Pract. 2020;20:101470.
- Sharma A, Pandit, V, Bhatia S, Kedia E, Bansod S. Chlorhexidine in caries prevention. International Journal of Current Research. 2019;11(12): 8979–8981.
- Colombo S, Beretta, M. Dental sealants part 3: which material? efficiency and effectiveness. Eur J Paediatr Dent. 2018;19:247–249.
- United States Centers for Disease Control and Prevention. Dental Sealants. Available at: cdc.gov/oralhealth/fast-facts/dental-sealants/index.html. Accessed April 22, 2022.
- United States Food and Drug Administration. Additional Information About High Intensity Sweeteners Permitted for Use in Food in the US. Available at: fda.gov/food/food-additives-petitions/additional-information-about-high-intensity-sweeteners-permitted-use-food-united-states. Accessed April 22, 2022.
- Nayak PA, Nayak UA, Khandelwal V. The effect of xylitol on dental caries and oral flora. Clin Cosmet Investig Dent. 2014;6:89–94.
- McComas M. Strategies to boost remineralization. Dimensions of Dental Hygiene. 2018;16(4):21–25.
- Zhao J, Liu Y, Sun W, Zhang H. Amorphous calcium phosphate and its application in dentistry. Chem Cent J. 2011;5:40.
- Burwell A, Litkowski L, Greenspan D. Calcium sodium phosphosilicate (NovaMin®): remineralization potential. Adv Dent Res. 2009;21:35–39.
- Somasundaram P, Vimala N, Mandke LG. Protective potential of casein phosphopeptide amorphous calcium phosphate containing paste on enamel surfaces. J Conserv Dent. 2013;16:152–156.
- Reynolds EC. Casein phosphopeptide-amorphous calcium phosphate: the scientific evidence. Adv Dent Res. 2009;21:25–29.
- Karlinsey R, Pfarrer A. Fluoride plus functionalized β-TCP: a promising combination for robust remineralization. Adv Dent Res. 2012;24:48–52.
- El-Ghannam AR. Advanced bioceramic composite for bone tissue engineering: design principles and structure–bioactivity relationship. J Biomed Mat Res. 2004;69:490–501.
- Li J, Huang Z, Mei L, Li G, Li H. Anti-caries effect of arginine-containing formulations in vivo: a systematic review and meta-analysis. Caries Res. 2015;49:606–617.
- University of Michigan. Naturally occurring amino acid could improve oral health. Available at: news.umich.edu/naturally-occurring-amino-acid-could-improve-oral-health. Accessed April 22, 2022.
- Wong C. What is L-arginine? Available at: verywellhealth.com/using-l-arginine-for-health-88322. Accessed April 22, 2022.
- Cummins D. The development and validation of a new technology based upon 1.5% arginine, and insoluble calcium compound and fluoride, for everyday use in the prevention and treatment of dental caries. J Dent. 2013;41S:S1–S11.
- Nascimento MM. Potential uses of arginine in dentistry. Adv Dent Res. 2018;29:98–103.
- Zheng X, He J, Wang L, et al. Ecological effect of arginine on oral microbiota. Sci Rep. 2017;7:7206.
- Bijle MNA, Ekambaram M, Lo ECM, et al. The combined antimicrobial effect of arginine and fluoride toothpaste. Sci Rep. 2019;9:8405.
- Chakraborty B, Burne RA. Effects of arginine on Streptococcus mutans growth, virulence gene expression, and stress tolerance. Appl Environ Microbiol. 2017;83:e00496–17.
- Tada A, Nakayama-Imaohji H, Yamasaki H, et al. Cleansing effect of acidic L-arginine on human oral biofilm. BMC Oral Health. 2016;16:40.
- Xue Y, Lu Q, Tian Y, Zhou X, Cheng L, Ren B. Effect of toothpaste containing arginine on dental plaque—a randomized controlled in situ study. J Dent. 2017;67:88–93.
- Nascimento MM, Browngardt C, Xiaohui X, Klepac-CeraJ V, Paster BJ, Burne RA. The effect of arginine on oral biofilm communities. Mol Oral Microbial. 2014;29:45–54.
- Kraivaphan P, Amornchat C, Triratana T, et al. Two-year caries clinical study of the efficacy of novel dentifrices containing 1.5% arginine, an insoluble calcium compound and 1,450 ppm fluoride. Caries Res. 2013;47:582–590.
- Yin W, Hu DY, Fan X, et al. A clinical investigation using quantitative light-induced fluorescence (QLF) of the anticaries efficacy of a dentifrice containing 1.5% arginine and 1,450 ppm fluoride as sodium monofluorophosphate. J Clin Dent. 2013;24(Spec no A):A15-A22.
- Medline Plus. L-Arginine. Available at: medlineplus.gov/druginfo/natural/_.html#Safety. Accessed April 22, 2022.
- Geraldeli S, Soares EF, Alvarez AJ, et al. A new arginine-based dental adhesive system: formulation, mechanical and anti-caries properties. J Dent. 2017;63:72–80.
- Griswold A, Chen Y, Snyder J, Burne R. 2004. Characterization of the arginine deiminase operon of Streptococcus rattus FA-1. Appl Environ Microbiol. 2004;70:1321–1327.
From Dimensions of Dental Hygiene. May 2022;20(5):21-25.

