
Separate the Facts from Fiction
Implementing the CDC “Guidelines for Infection Control in Dental Health-Care Settings” can be confusing. Here is straight talk on some of the most common misconceptions.
Proper infection control is necessary for the health and safety of both dental health care workers and patients. The United States Centers for Disease Control and Prevention (CDC) “Guidelines for Infection
Control in Dental Health-Care Settings—2003″1 are the gold standard for ensuring the provision of safe patient care. Gold standard status, however, does not ensure implementation. Clinicians may have questions about some aspects of the guidelines or feel reluctant to bring about change in established practice settings.
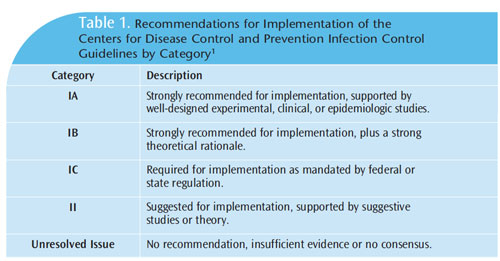
The CDC and the Healthcare Infection Control Practices Advisory Committee have provided recommendations for implementing the guidelines according to scientific theory, research, and application (Table 1).1 The categories range from “strongly recommended for implementation based on research and strong theoretical rationale” (IA and IB), “required implementation mandated by federal or state standards” (IC), to “no recommendation at all” (unresolved issue). When category IC (required) is used, a second rating can be added to provide the scientific basis for the recommendation.
Category II recommendations are supported by suggested studies or theory. Interpretation can vary if the recommendations are not well understood. Clinicians must understand which of the recommendations are required for implementation and which are suggestions or strong recommendations. Following are some common misconceptions about some of the guidelines’ recommendations, as well as a presentation of the research and rationale supporting them.
DISINFECTANT WIPES
In an effort to improve efficiency and contain costs, some clinicians try to make disposable disinfectant wipes by soaking 4 inch x 4 inch-sized gauze in a closed container with disinfectant. While this may seem like a creative idea, research indicates that the cotton fibers in gauze sponges can inactivate or absorb the chemicals in many surface disinfectants, rendering them useless against bloodborne pathogens.2 Therefore, disinfectants should not be stored in containers with gauze. If gauze is used to clean and wipe, then it should be saturated with the disinfecting agent at the time of use. Paper towels may be a more economical option.
Surface disinfectants are registered by the United States Environmental Protection Agency (EPA) and must be used according to the manufacturer’s directions. Intermediate level disinfectants (tuberculocidal claim) or the use of impervious barriers are required for clinical contact surfaces that are at risk of blood contamination.1 Commercial-grade wipes are convenient, but they are often used improperly. To correctly and effectively use wipes, the manufacturer’s instructions must be followed to the letter. Many wipes only cover a small surface area, so additional wipes may be needed to obtain minimal contact time. Remember, the surface area must first be cleaned of bioburden before a new wipe is used to disinfect.3 This method is known as “wipe-discard-wipe.”
Traditional liquid surface disinfectants and gauze or paper towels employ the same method and are familiar to most clinicians as the “spray-wipe-spray” technique.3 First, the surface is cleaned, and then it is disinfected. The CDC guidelines stipulate that all manufacturer’s instructions must be followed when using EPA-registered disinfection products (category IC), which would eliminate the possibility of creating wipes in-office. Category IB is also applicable because there is a scientific basis behind the recommendation.
STORING LOOSE INSTRUMENTS
Again, to maximize efficiency and reduce sterilization costs, some practices store instruments loose in drawers or on preset trays. The storage of sterile instruments varies according to their classification. Instruments are categorized as critical, semicritical, and noncritical. Critical instruments are those that penetrate bone or soft tissue, such as periodontal probes, curets, scalers, and oral surgery forceps.1 These instruments must be heat-sterilized and stored in a cool, dry area. Critical instruments are sterilized in a sealed wrap (pouch or cassette) and must not be opened until the point of use to avoid contamination and exposure to airborne pathogens and dust.1 Critical items should not be stored unwrapped.1 Wrapped instruments remain sterile until opened, unless the packaging has been compromised.1 Careful inspection of each package is necessary to ensure sterility. Critical instruments intended for immediate use may be sterilized unwrapped as long as sterility is maintained during transport from the sterilizer to the point of use in a sterile, covered container, and used immediately.1
Semicritical items enter the oral environment, but do not penetrate bone or soft tissue, such as mouth mirrors, anesthetic syringes, radiographic film/sensor holding devices, and reusable impression trays.1 These items should be heat-sterilized (if heat tolerant) or processed with high-level disinfection. If neither of these is possible, they should be covered with an impervious plastic barrier.1 Today, most semicritical items are heat tolerant.
Noncritical items contact skin surfaces or are exposed to oral fluids but do not enter the oral cavity, such as blood pressure cuffs, computer keyboards, and radiograph tube-head and exposure buttons. 1 Impervious barriers are routinely used for protecting these items.1
The CDC recommendation on the sterilization of critical instruments is category IC (required or mandated). As such, storing loose critical items in drawers or trays goes against the CDC guidelines, which state sterile critical items must remain packaged until the point of use. The only exception to this recommendation is when critical instruments intended for immediate use are sterilized and then immediately transferred to the point of use in a sterile, covered container. Semicritical items that will be used within a short time frame can be placed on a tray setup as long as they are handled aseptically.1 As there is a scientific evidence base supporting the need to keep critical items sterile, category IB is also applicable.
APPROPRIATE ATTIRE
Many clinicians are comfortable practicing in short-sleeved scrub tops, especially in a warm operatory. However, a protective, long-sleeved garment (labcoat or gown) must be worn by all clinicians while in the operatory. Forearms must be covered to protect skin from splash and spatter from blood, saliva, or other potentially infectious materials (OPIM).1 Short-sleeved scrubs can be worn underneath long-sleeved protective garments, which must be either disposable or laundered by the employer (or uniform service), according to the Occupational Safety and Health Administration.2,4 Long-sleeved protective garments should be changed if visibly soiled and should always be removed when leaving the work area.1,4 Wearing protective garments into nonclinical areas may lead to cross-contamination. Harmful pathogens, such as methicillin-resistant Staphylococcus aureus (MRSA), have been identified on uniforms of health care workers, but have not been implicated in disease transmission.5,6 Therefore, it is prudent to avoid wearing contaminated garments into any nonclinical areas, such as the lunch room, patient reception area, or outside of the office.
The CDC recommendation that requires that long-sleeved protective garments be worn in treatment areas is category IC (required or mandated). Category IB is also relative due to the availability of scientific evidence supporting the guideline.
UTILITY GLOVES
Although they may be unwieldly, puncture-resistant utility gloves are required when handling contaminated instruments to avoid sharps injuries.1 Ultrasonic cleaners, instrument washers, or cassettes are preferred methods of initial instrument handling and cleaning (prior to sterilization), as opposed to hand scrubbing. Sharps injuries are more likely to occur with hand scrubbing and should be avoided.1 Utility gloves must also be worn during operatory clean-up and setup when chemicals, such as surface disinfectants, are used.1 Protective eyewear, masks, and long sleeves should also be worn to avoid chemical splashes to mucous membranes (eyes, nose, and skin).1 This CDC recommendation is category IC (required or mandated) because the correct glove for the appropriate task is required when contact with chemicals, blood, or OPIM is likely. It also encompasses category IB as research shows the importance of using utility gloves.
HAND HYGIENE BEFORE AND AFTER GLOVE USE
The CDC recommendations require that clinicians don gloves when exposure to blood, bodily fluids, or OPIM is possible. The universal use of gloves in all health care settings has significantly reduced infection transmission in medical and dental settings. However, gloves are not an impermeable shield. Hands must be cleaned with either soap and water or alcohol-based hand rubs before donning gloves and after their removal. Transient microbial flora live on the superficial layers of the skin and are easily removed with handwashing or sanitizing. These transient organisms, such as MRSA, are generally responsible for infection outbreaks in hospitals. If hands are not washed prior to gloving, microorganisms on the skin thrive and rapidly multiply in the warm, moist environment of a glove. These microorganisms can then be transmitted because gloves do not provide a 100% barrier between the patient and clinician. Gloves have microscopic defects or pinholes through which pathogens can be transmitted during patient care, making the adherence to appropriate hand hygiene before and after glove use paramount to effective infection control policy. The guideline requiring effective hand hygiene before and after glove use is IC (required or mandated). Category 1B is also applicable due to evidence supporting this recommendation.
PREPROCEDURAL MOUTHRINSES
There is no consensus on whether the use of preprocedural mouthrinses is effective in reducing the amount microorganismladen aerosols released during patient treatment. The 2003 CDC guidelines do not support or refute the use of preprocedural rinsing, noting that the current science is unclear.1 The guidelines do state that antimicrobial preprocedural rinses, such as chlorhexidine gluconate, essential oils, or povidone iodine, are intended to reduce the number of microorganisms that might be released as aerosols or spatter during patient treatment, particularly when ultrasonic scalers and dental handpieces are used.1 Microorganismladen aerosols and spatter with the highest concentrations of micro organisms are typically located near the patient’s chest and the operator’s face, but they can linger in the air or settle on surfaces in the dental operatory and beyond.7 Suspended aerosols travel with air currents and have been found in treatment operatories for up to 2 hours after completion of dental procedures.8 Handpieces and ultrasonic scalers are used frequently by clinicians, so it may be prudent to incorporate preprocedural rinsing to reduce the amount of airborne microorganisms.
STERILIZATION OF SLOW-SPEED HANDPIECES
The issue of whether slow-speed dental hygiene handpieces need to be heat-sterilized after each patient is controversial. The CDC recommendations state that any item that can be removed from air or waterlines must be heat-sterilized after each use, because unintentional retraction of fluids into the internal surfaces could occur, causing cross-contamination. 1 Dye expulsion studies of high-speed handpieces have demonstrated fluid retraction (with the possibility of viral and bacterial materials) but have not been implicated in disease transmission.9–13
Flushing, lubricating, and heat sterilizing high-speed handpieces after each use is a general protocol for most clinicians. However, this issue has not been studied enough with slow-speed dental hygiene handpieces and ultrasonic scalers.1 These items are connected to air and waterlines, so why wouldn’t they be processed in the same way? The answer most likely comes down to cost and professional judgment. Most offices don’t have several slow-speed dental hygiene handpieces that can be swapped in while another is sterilized after each use.
There is no documented evidence in the literature linking these handpieces to cross-contamination or disease transmission, however, the CDC does recommend they be heat-sterilized after each use.1 The CDC recommendation that any item attached to air and waterlines must be heat-sterilized between patients is category IC. Until more research is conducted with slow-speed dental hygiene handpieces, the recommendation stands.
CONCLUSION
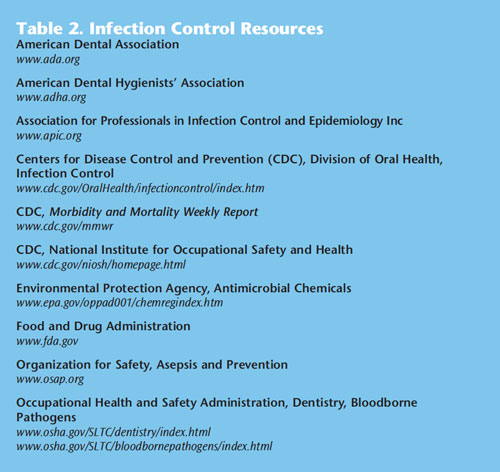
The 2003 CDC infection control guidelines are nearing their 10th anniversary. Denise Cardo, MD, director of the Division of Healthcare Quality Promotion at the CDC, reported at the 2012 annual Organization for Safety, Asepsis and Prevention (OSAP) symposium that the 2003 guidelines are currently in the second phase of being updated. This is a time-consuming and labor-intensive process due to the amount of evidence that must be reviewed. In order to ensure safety, clinicians should remain current with infection control protocol. Table 2 provides a list of infection control resources. OSAP offers numerous resources for dental professionals including membership, weekly email bulletins, quarterly newsletters, web resources and links, continuing education, and training resources. It is the responsibility of practitioners to consistently refresh their knowledge and stay abreast of changes. Clinicians should review their current infection control protocols with all key clinical staff members to determine if changes are needed based on professional judgment and the CDC infection control recommendations. Reviewing current office protocols with staff members is a chance to educate all team members (who may have varying degrees of infection control training), to elicit opinions, and empower group decision making. Compliance with infection control guidelines is enhanced when all team members are equally engaged in the decision-making process and have a stake in the development of office protocols.
REFERENCES
- Kohn WG, Collins AS, Cleveland JL, et al. Guidelines for Infection Control in Dental Health-Care Settings—2003. MMWR Recomm Rep. 2003;52:1–66.
- Molinari JA, Harte JA. Cottone’s Practical Infection Control in Dentistry. 3rd ed. Baltimore: Lippincott Williams& Wilkins; 2010.
- From Policies to Practice: OSAP’s Guide to the Guidelines: Workbook. Annapolis, Md: Organization for Safety,Asepsis and Prevention; 2004.
- Occupational Health and Safety Administration. Regulations (Standards 29 CFR). Available at: www.osha .gov/pls/oshaweb/owadisp.show_document?p_table=standards&p_id=10051. Accessed November 6, 2012.
- Kurita H, Kurashina K, Honda T. Nosocomial transmission of methicillin-resistant Staphylococcus aureus viathe surfaces of the dental operatory. Br Dent J. 2006;201:297–300.
- Wilson JA, Loveday HP, Hoffman PN, Pratt RJ. Uniform: an evidence review of the microbiologicalsignificance of uniforms and uniform policy in the prevention and control of healthcare-associated infections.Report to the Department of Health (England). J Hosp Infect. 2007;66:301–307.
- Leggat PA, Kedjarune U. Bacterial aerosols in the dental clinic: a review. Int Dent J. 2001;51:39–44.
- Dutil S, Meriaux A, Chantale de Latremoille M, Lazure L, Barbeau J, Duchaine C. Measurement of airbornebacteria and endotoxin generated during dental cleaning. J Occup Environ Hyg. 2009;6:121–130.
- Crawford JJ, Broderius C. Control of cross-infection risks in the dental operatory: prevention of waterretraction by bur cooling spray systems. J Am Dent Assoc. 1988;116:685–687.
- Mills SE, Kuehne JC, Bradley DV Jr. Bacteriological analysis of high-speed handpiece turbines. J Am DentAssoc. 1993;124:59–62.
- Lewis DL, Arens M, Appleton SS, et al. Cross-contamination potential with dental equipment. Lancet.1992;340:1252–1254.
- Lewis DL, Boe RK. Cross-infection risks associated with current procedures for using high-speed dentalhandpieces. J Clin Microbiol. 1992;30:401–406.
- Checchi L, Montebugnoli L, Samaritani S. Contamination of the turbine air chamber: a risk of crossinfection. J Clin Periodontol. 1998;25:607–611.
From Dimensions of Dental Hygiene. December 2012; 10(12): 44–48.

