CE Sponsored by Colgate in Partnership with the American Academy of Periodontology: Effects of Aging on Periodontal Health
The cumulative effects of periodontal diseases make it difficult to distinguish long standing disease from age related incidence of disease.
This course was published in the July 2017 issue and expires July 2020. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Discuss the age-related changes of the periodontium that occur at the cellular and tissue level.
- Compare and contrast the two hypotheses—the cumulative effect hypothesis and age-altered susceptibility hypothesis—that may explain older adults’ susceptibility to periodontal diseases.
- Identify the age-related changes of immune responses that may affect periodontal health.
- List the age-related changes of gingival microbial composition.
- Explain the age-related changes in clinical periodontal parameters.
Introduction
As health care professionals, dental team members must take into account the changes to the oral cavity that are caused by the normal aging process in older adult patients. Understanding these age-related changes plays an important role when determining a long-term prevention program, which is often individualized to patients’ needs with the goal of helping them maintain optimal oral health. This article “Effects of Aging on Periodontal Health” reviews the periodontal changes to cells, immune response, and gingival composition caused by age. I hope you find this article to be a valuable resource to help you manage older adult patients. The Colgate-Palmolive Company is delighted to have provided an unrestricted educational grant to support this continuing education article series.
—Matilde Hernandez, DDS, MS, MBA
Scientific Affairs Manager
Colgate Oral Pharmaceuticals
Clinicians need to discuss the changes to periodontal health that accompany aging with their older adult patients and reinforce preventive/protective measures to support periodontal health.
FROM THE AMERICAN ACADEMY OF PERIODONTOLOGY
In 2012, the United States Centers for Disease Control and Prevention found that more than 70% of the nation’s periodontal disease cases occur in people age 65 and older, suggesting a direct correlation between periodontal disease risk and age. The expression of being “long in the tooth” is often used to describe an older person, as if the gum disease and receding gums that signify it are a fact of aging. But they don’t have to be. In this article, educator and American Academy of Periodontology (AAP) periodontist Sivaraman Prakasam, BDS, MSD, PhD, discusses the periodontal changes that may occur in aging populations and how oral health professionals are key in educating patients about methods of prevention and care. The AAP is proud to work with Dimensions of Dental Hygiene and Colgate-Palmolive to bring you insights that will keep your patients healthy at every age.
—Terrence J. Griffin, DMDPresident, American Academy of Periodontology
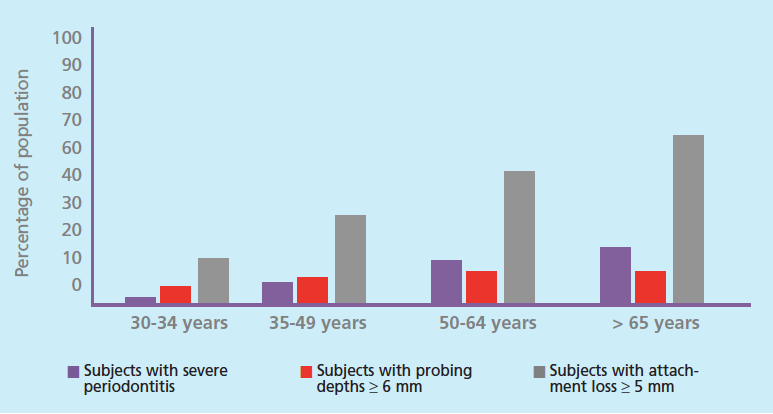
The patient populations of today’s oral health professionals are increasingly older. At the end of the next decade, one out of five adults in the United States are projected to be age 65 or older.1 Most of these adults will retain at least some of their teeth. For example, in 2012, only 13.7% of US adults age 65 to 74 were edentulous, while 55.4% of adults age 65 to 74 were edentulous in 1958.2 This trend of tooth retention is a direct result of advances in oral health care, but an emerging challenge is facing dental and health care systems: the increasing prevalence of chronic diseases. For example, 70% of older adults (age 65 and older) experience periodontitis, of which 64.2% have moderate to severe forms (Figure 1).3
While the high prevalence of periodontal diseases in the US is shocking, one possible explanation is that periodontal diseases are cumulative in nature. The progression of periodontal diseases hinders clinicians’ abilities to clinically distinguish the changes that are part of the normal aging process from changes that are indicative of the pathologic process.4 Aging causes physiological changes in the tissues, organs, and systems. These changes occur at different rates and are influenced by several factors, such as lifestyle, environment, and genetics.4 When the effects of these changes result in loss of tooth structure and/or function, they cross into pathologic processes. In this framework, determining between the effects of aging on the periodontium vs disease progression that needs treatment is challenging.
CELLS AND TISSUES OF THE PERIODONTIUM
The different components of the periodontal-attachment apparatus undergo physiological aging. Gingival epithelium becomes thin and has less keratinization with progressing age.5 Furthermore, some reports suggest that alterations of cell density and flattening of rete pegs (epithelial extensions that protrude into the connective tissue in skin and mucous membranes) may be common during the aging process.6 These changes reduce the barrier ability of the epithelium. Junctional epithelium, on the other hand, migrates to a more apical position along with clinical signs of gingival recession.7 Interestingly, this migration doesn’t result in decreased width of attached gingiva.8 The functional consequence of this apical migration is not known, but it’s thought that it may be the result of passive eruption of teeth to compensate for occlusal wear.
The gingival connective tissue becomes coarse and denser with age, likely due to collagen stabilization caused by changes in macromolecular conformation.9 The actual rate of collagen synthesis itself decreases with age.6,10 These changes make the gingiva less elastic and hence less resilient to injury. The periodontal ligament undergoes a reduction in fibroblasts and becomes more irregular with age.5,6 This change likely affects its healing capability. Data on the effect of aging on the width of the periodontal ligament are not clear, as this may be more dependent on functional aspects of the tooth than age.5
Cementum continues to increase with age and is usually three times to four times thicker in older adults.11 Similar to apical migration of junctional epithelium, the increase in cementum thickness is likely the result of passive eruption of teeth to maintain lost occlusal contact and is secondary to tooth wear. Alveolar bone, in proximity to the periodontal-attachment apparatus, appears to have more irregular bone surface and irregular insertion of collagen fibers. Alveolar bone also shows a decrease in bone density, increase in bone resorption, and a decrease of vascularity with increasing age.5 These changes suggest less tolerance of alveolar bone to injury among older individuals.
PERIODONTAL IMMUNE RESPONSES
In general, physiological loss of periodontal attachment due to aging is minimal. Nevertheless, the cumulative effects of long-standing microbial insult and resultant cumulative inflammatory damage may explain the increased prevalence of periodontal diseases observed in older age groups. Hajishengallis12 described this as the “cumulative effect hypothesis.” On the other hand, aging may cause alterations to the immuno-inflammatory status of the periodontal tissue. This, in turn, may increase susceptibility to periodontitis. Hajishengallis12 describes this alternative hypothesis as “age-altered susceptibility hypothesis.”
Some clinical evidence (studies that used experimental gingivitis study design) exists to support the age-altered susceptibility hypothesis. Young adults (age 20 to 25) and older adults (≥ 65) were given professional dental care to establish a baseline of gingival health. They were then asked to abstain from oral hygiene measures for 3 weeks. Both groups had comparable dental biofilm accumulation. Interestingly, the older individuals had more severe gingivitis associated with increased accumulation of inflammatory cells when compared with the younger adults.13 A similar experimental gingivitis study showed that the older adults had significantly higher levels of interleukin 1 beta (IL-1β) in gingival crevicular fluid as compared with the young adults at the end of 3 weeks of abstinence from oral hygiene.14 The biological reasons for these observations, however, remain unclear.
Age-related alterations may affect both arms of the immune system, namely the innate immune response and the adaptive immune response. Adaptive or acquired immune responses are pathogen specific. This type of immune response gradually declines with age.15 This occurs due to decreased production of white blood cells, such as naïve T cells (specific type of white blood cells that have not been exposed to pathogen components) and B lymphoid progenitor cells.15 Other alterations include:
- Defects in cell signal transduction mechanisms and altered cytokine induction patterns (defects in defense response of T cells).
- Reduction in expansion of clones of antigen specific T and B cells (no increase in cells that target specific antigens, eg, bacterial cell wall components). The reduction in naïve T cells is due to shrinking of the thymus with age. The reduction in B lymphoid progenitor cells is likely due to skewing of hematopoietic stem cells toward a more myeloid lineage with age.15,16
Innate immune responses, on the other hand, are the first line of defense that are germline encoded. These responses do not require previous exposure to specific pathogens or antigens. Age-related alterations of the innate immune mechanisms do not necessarily cause immunodeficiency. These changes may result in dysregulation or alteration of the host immune response, in other words, either increasing or decreasing immune activity.17 The key cells involved in innate immune responses are usually neutrophils, monocytes/macrophages, and dendritic cells.
The total number of circulating neutrophils does not decrease with age.18 The receptors that are normally present are also not reduced with age. Additionally, neutrophils in older adults maintain the expression of adhesion molecules and are able to adhere to the endothelial cells that line blood vessels. However, neutrophils become less effective in chemotaxis as individuals age and cannot migrate in response to stimuli compared with neutrophils in young adults. They are also inefficient in phagocytosis and microbicidal activity.19,20 Older neutrophils experience a reduction in the production of reactive oxygen species.19,20 With age, they also have several deficiencies in signal transduction activity.21 They also undergo increased programmed cell death in response to stimuli and secrete increased levels of cytokine-signaling inhibitory molecules.22 Overall, these changes reduce the innate immune response of neutrophils to plaque biofilm.
Like neutrophils, monocytes/macrophages maintain the receptors necessary for recognizing pathogens during the aging process. They do experience deficiencies in signal transduction mechanisms, resulting in reduced cytokine production.20 Additionally, monocytes/macrophages in older adults have a reduced ability to phagocytose, produce reactive oxygen species, and create reactive nitrogen species.23 They are less able to do intracellular killing as well as express specific innate immune receptors. Interestingly, they produce higher levels of prostaglandin E2.24 Overall, monocytes/macrophages become less efficient in innate immune response with age.
With age, dendritic cells increase production of inflammatory cytokines like tumor necrosis factor-alpha (TNF-α) and IL-6.25 They also increase basal expression of co-stimulatory molecules. In contrast, they are less effective in their antigen presentation ability (ability to link innate immunity to the adaptive immune response).26 Their ability to undergo chemotaxis and endocytosis is compromised.27Dendritic cells do retain their ability to produce an inflammatory response to innate mechanisms but are less able to present the antigens to the adaptive immunity.
While several studies have looked at the innate functions of cells and their alteration with age, most are in vitro studies in which the cells are isolated from their native tissue environment.12 Tissues may not be passive recipients of immune surveillance and may have greater control in modulating the host response and providing homeostatic protection against inflammatory diseases.12
GINGIVAL MICROBIAL COMPOSITION
The subgingival microbial composition of older adults was first analyzed by Newman et al28 in 1978. They noted a predominance of Gram-positive, aerobic bacteria and the presence of relatively small numbers of Gram-negative anaerobes. Percival et al29 compared the microbial composition of saliva and supragingival biofilm among several age groups using culturing techniques. They didn’t observe any differences in salivary microbial composition between the age groups. They did observe statistically higher numbers of Actinomyces species in supragingival biofilms among older adults.29 Similar results were reported by Marsh et al.30 Preza et al31 looked at bacterial diversity in various oral niches in older adults. They reported a higher diversity of species in periodontally healthy older adults compared with young and middle-aged periodontally healthy adults. Rodenburg et al,32 on the other hand, looked at the prevalence of periodontal pathogens in subjects with untreated or refractory periodontitis as a function of age. They noted that the prevalence of Aggregatibacter Actinomycetemcomitansdecreased with age while an increase was noted in the prevalence of Porphyromonas gingivalis.32 This has been observed by others, as well.33–35 The concept that older subjects with refractory periodontitis may harbor superinfecting microorganisms has been proposed by Slots et al.36 They showed an increased prevalence of enteric rods and Pseudomonas species compared with young individuals.36
A recent study reported that the composition of subgingival biofilms was similar across age groups and was more dependent on disease status than on age.37 This study also showed a higher prevalence of Actinomyces sp. in older adults.37 The authors proposed that the presence of increased root surfaces and/or prosthetic surfaces in older adults may offer a selective advantage for these bacteria.37 Based on these studies, it seems that microbial composition typically remains similar across all age groups. Nevertheless, the functional aspects—or metabolomics—of the microbial community may be different in older adults and this is currently being explored with modern microbiological techniques.
CLINICAL PERIODONTAL PARAMETERS
Attachment loss is often considered to be caused by and evidence of periodontal diseases. Arguably, the aging process by itself can result in a modest reduction in periodontal attachment apparatus (Figure 2). Lamster et al4 suggest that gingival recession of 3 mm or less on buccal surfaces may be the result of normal aging, with the caveat that > 3 mm loss can still be considered physiological if a tooth is functional, has no mobility, and no discomfort is present. They suggest that some increase in crown-to-root ratio is common with healthy aging, but teeth will have only physiological mobility and probing depths ≤ 4 mm. They acknowledge that an alternative explanation for this age-related attachment could be the result of repeated use and low-level insult over decades.4
Epidemiological analysis highlights the difficulty in distinguishing cumulative effects of disease process with age-related effects on periodontal health. A global review of epidemiological data from 1990 to 2010 on the prevalence of severe chronic periodontitis (pocket depths > 5 mm) suggests that the prevalence of severe disease increases sharply in subjects between the ages of 20 and 40, with a peak incidence at age 38, followed by gradually plateauing of prevalence.38 Using the same criteria, an analysis of US population by Eke et al,3 shows the prevalence of periodontal diseases is almost double among adults older than 50 (15.8%) than adults age 35 to 49 (8.1%). They also report that the prevalence of severe attachment loss > 5 mm steadily increases with age (Figure 1). The prevalence of mean probing depths > 6 mm also show a steady albeit less dramatic increase ranging from 5.9% (ages 30-34) to 11.9% (ages > 65).3
CLINICAL RELEVANCE
The World Health Organization recommends the use of therapeutic patient education (TPE) in the management of chronic diseases. TPE focuses on discussing the conditions, in terms of knowledge, skills, and attitudes, in addition to the support needed in each case to promote dialogue and consensus.39 The use of this type of education in managing/preventing periodontal diseases is particularly relevant for older patients. Consequently, understanding age-related changes and their impact on the periodontium helps clinicians discuss these changes with older adults and reinforce the importance of preventive/protective measures.
Clinicians should educate older adult patients about the implications of the loss of innate cellular/tissue level defense mechanisms, as well as the negative impact on the effectiveness of the immune response with age. As with patients of any age, older adults need to be provided with patient-tailored oral hygiene instructions. These instructions should be reinforced at every preventive/maintenance appointment. The use of antimicrobial self-care products, including dentifrice, and oral hygiene aids should be recommended to patients based on their individual needs. A Swedish study suggests that routine dental care alone is not sufficient to prevent progression of periodontitis in older adults.40 Consequently, the necessity of an effective oral hygiene regimen must be consistently emphasized.
Older adults are at increased risk for several systemic diseases that also affect their oral health status and care.39 Understanding the impact of these diseases and the effects of aging on the periodontium helps clinicians provide the most appropriate periodontal care for older adults.39,41
SUMMARY
Aging impacts the periodontium and how it relates to health and disease. With age, the cells and tissues that form the periodontal attachment apparatus undergo various compensatory changes, the majority of which negatively affect the innate protection provided by these cells and tissues to various insults. The adaptive immune mechanism becomes less effective with age. The innate immune mechanism either becomes less effective or hyper inflammatory, depending on the cellular context. Microbial composition overall remains the same and is perhaps more diverse in older adults with healthy periodontium. Clinical changes for the most part are minimal in healthy older adults, but cumulative effects of disease make it difficult to distinguish long-standing disease from age-related incidence of disease.
Clinicians need to discuss these changes with their older adult patients and reinforce preventive/protective measures. In addition, understanding age-related changes to periodontal health will guide clinicians in the adjustment of periodontal care for older adults using existing frameworks of care.39
References
- United States Census Bureau. The next fourdecades: the older population in the United States: 2010 to 2050. Available at:census.gov/prod/2010pubs/p25-1138.pdf. Accessed June 25, 2017
- Slade G, Akinkugbe A, Sanders A.Projections of US edentulism prevalence following 5 decades of decline. J Dent Res.2014;93:959–965.
- Eke P, Dye BA, Wei L, et al. Update onprevalence of periodontitis in adults in the United States: NHANES 2009 to2012. J Periodontol. 2015;86:611–622.
- Lamster IB, Asadourian L, Del Carmen T,Friedman PK. The aging mouth: differentiating normal aging from disease. Periodontol 2000.2016;72:96–107.
- Bhadbhade S. Aging and periodontium. Int J Dent Oral Sci. 2015;2:79–83.
- Berglundh T, Lindhe J, Sterrett J.Clinical and structural characteristics of periodontal tissues in young and olddogs. J Clin Periodontol. 1991;18:616–623.
- Lindhe J, Hamp SE, Löe H. Plaque inducedperiodontal disease in beagle dogs. J Periodontol Res. 1975;10:243–255.
- Ainamo A, Ainamo J, Poikkeus R. Continuouswidening of the band of attached gingiva from 23 to 65 years of age. J Periodontol Res. 1991;16:595-599.
- Wentz FM, Maier AW, Orban B. Age changesand sex differences in the clinically “normal” gingiva. J Periodontol.1952;23:13–24.
- Ellen RP. Periodontal disease among olderadults: what is the issue? Periodontol 2000. 1998;16:7–8.
- Zander HA, Hürzeler B. Continuous cementumapposition. J Dent Res. 1958;37:1035–1044.
- Hajishengallis G. Aging and its impact oninnate immunity and inflammation: implications for periodontitis. J Oral Biosc.2014;56:30–37.
- Fransson C, Berglundh T, Lindhe J. Theeffect of age on the development of gingivitis. J Clin Periodontol. 1996;23:379–385.
- Tsalikis L, Parapanisiou E, Bata-Kyrkou A,Polymenides Z, Konstantinidis A. Crevicular fluid levels of interleukin-1alphaand interleukin-1beta during experimental gingivitis in young and old adults. J Int Acad Periodontol. 2002;4:5.
- Weng N. Aging of the immune system: howmuch can the adaptive immune system adapt? Immunity. 2006;24:495–499.
- Geiger H, De Haan G, Florian MC. Theageing haematopoietic stem cell compartment. Nat Rev Immunol. 2013;13:376–389.
- Gomez CR, Nomellini V, Faunce DE, KovacsEJ. Innate immunity and aging. Exp Gerontol. 2008;43:718-728.
- Solana R, Tarazona R, Gayoso I, Lesur O,Dupuis G, Innate TF. Immunosenescence: Effect of aging on cells and receptorsof the innate immune system in humans. Semin Immunol. 2012;24:331–341.
- Wenisch C, Patruta S, Daxböck F, Krause R,Hörl W. Effect of age on human neutrophil function. J Leukoc Biol.2000;67:40-45.
- Mahbub S, Brubaker AL, Kovacs EJ. Aging ofthe innate immune system: an update. Curr Immunol Rev. 2011;7:104–115.
- Tortorella C, Simone O, Piazzolla G,Stella I, Antonaci S. Age-related impairment of GM-CSF-induced signalling inneutrophils: role of SHP-1 and SOCS proteins. Ageing Res Rev. 2007;6:81–93.
- Larbi A, Douziech N, Fortin C, Linteau A,Dupuis G, Fulop T Jr. The role of the MAPK pathway alterations in GM-CSFmodulated human neutrophil apoptosis with aging. Immun Ageing.2005;2:6.
- Stout RD, Suttles J. Immunosenescence andmacrophages functional plasticity: dysregulation of macrophage function by age?associatedmicroenvironmental changes. Immunol Rev. 2005;205:60–71.
- Kovacs EJ, Palmer JL, Fortin CF, Fülöp TJr, Goldstein DR, Linton PJ. Aging and innate immunity in the mouse: impact ofintrinsic and extrinsic factors. Trends Immunol. 2009;30:319–324.
- Panda A, Qian F, Mohanty S, et al.Age-associated decrease in TLR function in primary human dendritic cellspredicts influenza vaccine response. J Immunol. 2010;184:2518–2527.
- Sridharan A, Esposo M, Kaushal K, et al.Age-associated impaired plasmacytoid dendritic cell functions lead to decreasedCD4 and CD8 T cell immunity. Age (Dordr). 2011;33:363–376.
- Agrawal A, Agrawal S, Cao JN, Su H, OsannK, Gupta S. Altered innate immune functioning of dendritic cells in elderlyhumans: a role of phosphoinositide 3-kinase-signaling pathway. J Immunol. 2007;178:6912–6922.
- Newman MG, Grinenco V, Weiner M, Angel I,Karge H, Nisengard R. Predominant microbiota associated with periodontal healthin the aged. J Periodontol. 1978;49:553–559.
- Percival R, Challacombe S, Marsh P.Age-related microbiological changes in the salivary and plaque microflora ofhealthy adults. J Med Microbiol. 1991;35:5–11.
- Marsh P, Percival R, Challacombe S. Theinfluence of denture-wearing and age on the oral microflora. J Dent Res.1992;71,:1374–1381.
- Preza D, Olsen I, Willumsen T, et al.Microarray analysis of the microflora of root caries in elderly. Eur J Clin Microbiol Infect Dis. 2009;28:509.
- Rodenburg JP, van Winkelhoff AJ, WinkelEG, Goené RJ, Abbas F, de Graff J. Occurrence of bacteroides gingivalis,Bacteroides intermedius and Actinobacillus actinomycetemcomitans in severeperiodontitis in relation to age and treatment history. J Clin Periodontol. 1990;17:392–399.
- Savitt ED, Kent RL. Distribution ofActinobacillus actinomycetemcomitans and Porphyromonasgingivalis bysubject age. J Periodontol. 1991;62:490–494.
- Slots J, Feik D, Rams TE. Actinobacillus actinomycetemcomitans and Bacteroidesintermediusinhuman periodontitis: age relationship and mutual association. J Clin Periodontol. 1990;17:659–662.
- Teixeira SR, Mattarazo F, Feres M, et al.Quantification of Porphyromonas gingivalis and fimA genotypes in smoker chronicperiodontitis. J Clin Periodontol. 2009;36:482–487.
- Slots J, Feik D, Rams TE. Age and sexrelationships of superinfecting microorganisms in periodontitis patients. Oral Microbiol Immunol. 1990;5:305–308.
- Feres M, Teles F, Teles R, Figueiredo LC,Faveri M. The subgingival periodontal microbiota of the aging mouth. Periodontol 2000.2016;72:30–53.
- Kassebaum NJ, Bernabé E, Dahiya M,Bhandari B, Murray CJ, Marcenes W. Global burden of severe periodontitis in1990-2010: a systematic review and meta-regression. J Dent Res.2014;93:1045–1053.
- Prakasam S. Periodontal care for olderadults. Dimensions of Dental Hygiene. 2015;13:45–50.
- Nordström G, Bergman B, Borg K, Nilsson H,Tillberg A, Wenslöv JH. A 9-year longitudinal study of reported oral problemsand dental and periodontal status in 70- and 79-year-old city cohorts innorthern Sweden. Acta Odontol Scand. 1998;56,:76–84.
- Ettinger R. Treatment planning conceptsfor the ageing patient. Aust Dent J. 2015;60:71–85.
Featured photo by DANCHOOALEX/E+/GETTY IMAGES PLUS
From Dimensions of Dental Hygiene. July 2017;15(7):45-50.



