 MCBWORLD/ISTOCK/THINKSTOCK
MCBWORLD/ISTOCK/THINKSTOCK
Addressing Obstructive Sleep Apnea
Oral health professionals play important roles in detecting and treating obstructive sleep apnea.
This course was published in the January 2017 issue and expires January 2020. The author has no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Define obstructive sleep apnea (OSA).
- Discuss the risk factors and conditions associated with this sleep disorder.
- Recognize OSA’s characteristics and clinical features.
- Identify screening questionnaires and classifications to assess the risk for sleep disorders.
- Describe diagnosis and treatment options for patients with OSA.
The severity of OSA is based on the apnea-hypopnea index (AHI) and/or the respiratory disturbance index (RDI). The AHI is the combined average of apneas and hypopneas occurring in 1 hour. The RDI is the number of apneas, hypopneas, and respiratory-related arousals occurring in 1 hour. Mild RDI/AHI is five arousals to 15 arousals per hour; moderate is 15 arousals to 30 arousals per hour; and severe is more than 30 arousals per hour.3
Oral health professionals are well positioned to identify and provide treatment to patients with OSA. Additionally, interprofessional collaboration with patients’ medical and dental teams can help maximize their quality of life. Physicians can detect OSA, prescribe a sleep study, make the diagnosis, and determine the treatment plan. Oral health professionals can identify risk factors, make appropriate referrals if OSA is suspected, and treat patients with OSA by fabricating an oral appliance. Specifically, dental hygienists can screen for risk factors and clinical signs, as well as provide screening questionnaires to patients.
RISK FACTORS
Risk factors for OSA vary from a high body mass index (BMI) to a history of tobacco use. Adults with a high BMI (≥30 kg/m2) and large neck circumference (>17 inches for men, >16 inches for women) is one of the greatest predictors of OSA.3 OSA can be present, however, in patients with any BMI and/or neck circumferences.
Age is another risk factor for OSA. The Sleep Heart Health Study, a multicenter cohort study funded by the National Health Lung & Blood Institute, investigated the negative effects of sleep-disordered breathing on cardiovascular and other health systems. The study included 6,441 men and women age 40 or older. Results showed that as participants aged, their risk of OSA increased.4
Men are at increased risk of OSA compared with women. The Wisconsin Sleep Cohort Study estimated the prevalence of OSA in middle-aged adults age 30 to 60 at 9% in women and 24% in men. However, this disparity diminishes when women experience hormonal and physiological changes (eg, menopause).5
Sleep position also plays a role in OSA prevalence. Research shows that adults with positional OSA had double the number of obstructive events when sleeping in the supine position than when sleeping in the lateral position.6 Generally, adults who have positional OSA are younger and have a lower BMI than those unaffected by sleep position.6
Consuming alcohol can raise OSA risk. Men who drink at least one alcoholic drink per day are 25% more likely to experience OSA than men who do not.7 Additionally, research suggests that drinking alcohol before bed may acutely impact breathing patterns while sleeping.8 Adults who use tobacco are also four times more likely to develop moderate/severe OSA than nontobacco users.9
If OSA is left untreated, the risk for serious health problems, including death, increases. Diabetes is related to OSA, with 70% of adults with type 2 diabetes experiencing OSA.10 The severity of untreated OSA negatively impacts glucose levels.
Patients with hypertension are 30% to 40% more likely to develop OSA than those without hypertension.10 The more severe the patient’s OSA, the more likely he or she is to experience hypertension.11 The risk for stroke and death in patients with OSA is almost double than for those without OSA.12
Oral health problems are more common among patients with OSA. While it is a new area of investigation, increasing evidence demonstrates an association between chronic periodontitis and OSA. Adults with moderate and severe chronic periodontitis are four times more likely to develop OSA than adults with mild chronic periodontitis and gingivitis.13 Adults at high risk for OSA were 73% more likely to develop first-onset temporomandibular joint disorder (TMJ) than individuals at low risk of OSA.14 A study also showed that 52% of individuals with mild to moderate OSA presented with signs and symptoms of TMD.15 Sleep bruxism is another oral health problem related to OSA that may serve as a compensating behavior through the pushing of the mandible forward in an attempt to open the airway.16 Approximately 25% of patients with OSA exhibit sleep bruxism, compared with 5% to 8% in the general population.17
CHARACTERISTICS AND CLINICAL SIGNS
Characteristics commonly associated with OSA include snoring, witnessed cessation of breathing by a bed partner, and excessive daytime sleepiness. Specifically, excessive daytime sleepiness (self-report) has been found in 22.6% of women and 15.5% of men with OSA.5
Large tonsils, long uvulas, large tongues, and a crowded pharyngeal space are associated with OSA.18 Large tonsils create a small airway, which is more easily obstructed. A large tongue rests on top of or above the mandibular teeth, causing airflow obstruction when it falls back into the throat during sleep. A thick, broad, and scalloped tongue may be a sign of OSA. A crowded pharyngeal space limits the amount of airflow. Additional signs of OSA are dry mouth, narrow palate, anterior attrition, and retrognathia.19
SCREENING TOOLS
Screening tools can assess patients’ risk of OSA and should be included in the medical history and intraoral exam. Numerous screening questionnaires are available. The four most commonly used in the dental setting are the STOP Questionnaire,STOP-Bang Questionnaire, Mallampati Airway Classification, and Epworth Sleepiness Scale.20–23
 In 2008, Chung et al20 created the STOP Questionnaire—an easy-to-use, self-administered questionnaire designed to measure an individual’s risk for OSA (Table 1). If a patient answers “yes” to two or more questions, he or she is considered high risk for OSA.
In 2008, Chung et al20 created the STOP Questionnaire—an easy-to-use, self-administered questionnaire designed to measure an individual’s risk for OSA (Table 1). If a patient answers “yes” to two or more questions, he or she is considered high risk for OSA.
The STOP-Bang Questionnaire is an alternative risk assessment that adds questions regarding BMI (more than 35 kg/m2), age (50 or older), neck circumference (greater than 40 cm), and gender (male) to the STOP Questionnaire. If patients answer yes to three or more items, they are considered at risk for OSA.20
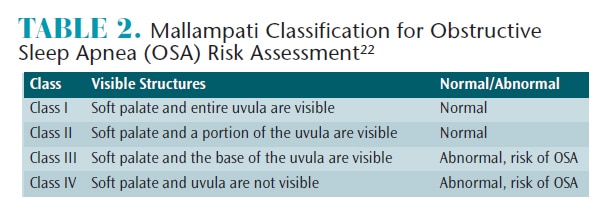
The Mallampati Airway Classification was created in 1985 to help predict the ease of tracheal intubation for patients undergoing surgery.21 More recently, the Mallampati classification has been used to assess OSA risk.22 During the assessment, patients are instructed to open their mouths and protrude their tongues while in a seated position. The oral health professional grades the airway based on four classes (Table 2). Classes III and IV indicate high risk for OSA.22
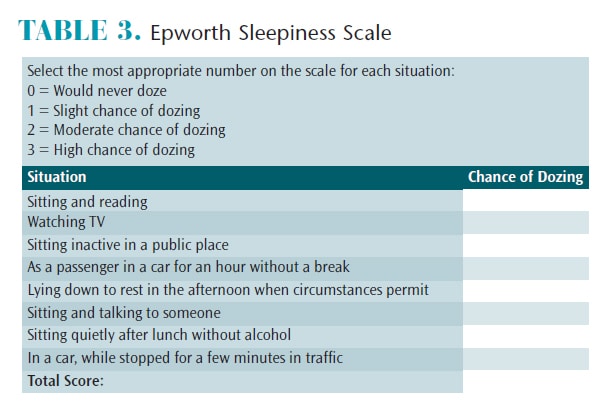
Johns23 developed the Epworth Sleepiness Scale (ESS) in 1991. It is a self-administered questionnaire that assesses daytime sleepiness. Patients rate their chances of dozing in eight scenarios and the scores are added (Table 3). An ESS score of 16 or higher is considered a high level of daytime sleepiness.23
DIAGNOSIS AND TREATMENT
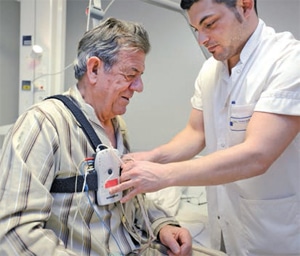
The definitive diagnosis of OSA is made through polysomnography, an overnight sleep study (Figure 1). A polysomnography is monitored by a sleep technician throughout the night and interpreted by a sleep physician once the study is completed. It monitors a patient’s blood oxygen level, heart rate, brain waves, breathing, and eye and leg movements during sleep.24 Once the sleep physician interprets the results, he or she determines the diagnosis and severity of the sleep disorder.
OSA treatment typically requires a long-term, multidisciplinary approach. Treatment regimens vary based on the patient’s needs, anatomy, and preferences. Treatment options include: positive airway pressure (PAP), behavioral modifications, surgery, orofacial myofunctional therapy, and oral appliances.3
PAP is the first treatment of choice for patients with OSA. It is a long-term treatment that keeps the airway open via air pressure while sleeping. It can be delivered through continuous positive airway pressure (CPAP), bilevel positive airway pressure (BPAP or BiPAP), or automatic, self-adjusting airway pressure (APAP). A PAP machine uses a mask that either fits over the nose or nose and mouth, a tube that connects the mask to the machine’s motor, and a motor that blows air into the tube to boost airway flow. Today’s PAP machines are lightweight, fairly quiet, and have a success rate of 80% to 95%.25Common challenges are mask leaks, loud noise (although this has improved over the years), nasal congestion, xerostomia, claustrophobia, irritation from the mask and straps, and too much air pressure.26 These challenges can lead to noncompliance and cessation of treatment.
Behavioral modifications include weight loss (ideal BMI: 25 kg/m2 or less), exercise, avoiding alcohol and sedatives before sleep, positional therapy,3 and smoking cessation.8 Surgeries may include uvulopalatopharyngoplasty, tonsillectomy, adenoidectomy, genioglossus advancement, telegnathic maxillomandibular advancement surgery, or a combination. Orofacial myofunctional therapy may be added in conjunction with other OSA treatments and consists of exercises that involve the lip, tongue, soft palate, and pharyngeal wall.27
If a patient cannot tolerate PAP, or snores, the physician may refer the patient to an oral health professional who is certified to fabricate and adjust an oral appliance.28 Once referred, the oral health professional will evaluate the patient’s oral cavity. The patient must receive dental treatment if active disease is present (periodontitis, caries lesions) before an oral appliance is made. Oral appliance options consist of mandibular advancement splints (MAS) and tongue-retaining devices (TRD). A MAS engages the mandible and the tongue (indirectly), repositioning the mandible in a forward position. It is made of two acrylic resin pieces covering the maxillary and mandibular arches. An additional part connects two pieces to hold the mandible forward. Appliances can either be titratable or nontitratable. Titratable appliances allow for adjustments to the degree of mandibular protrusion during use, while nontitratable appliances keep the mandible in a fixed state of protrusion.28 Side effects that occur within the first few days or weeks of MAS therapy are typically minor and may include masticatory muscle or temporomandibular joint discomfort. Side effects occurring 6 months or later after the start of therapy typically result in cessation of the treatment and may include changes in tooth position and occlusion. An aligner should be fabricated in order to avoid these changes in tooth position and occlusion.
TRDs directly engage the tongue and hold it in a forward position. They are generally made of silicone and are in the shape of a bulb or cavity. The bulb produces a suction that holds the tongue forward in the bulb. Tongue-retaining devices full breath solution (FBS) are single-arch oral sleep appliances and use a tail that depresses and restrains the tongue. Once an oral appliance is made, a polysomnography needs to be completed in order to ensure the oral appliance is effective. The average success rate for oral appliances is 52%. This treatment option should be reserved for those patients with severe OSA who did not benefit from CPAP therapy.23

ROLE OF DENTAL HYGIENISTS
Physicians are the only health professionals who can diagnose OSA. Oral health professionals, however, play a pivotal role in detection and treatment of OSA and work interprofessionally with the sleep medicine team. Oral health professionals may be the first to detect signs of OSA or sleep problems, and physicians may refer a patient to a dentist for fabrication of an oral appliance. Dental hygienists specifically can:
- Screen patients for OSA by checking signs of bruxism and OSA and including OSA screening questionnaires in medical histories.
- Refer patients to their physicians if OSA is suspected.
- Screen patients for an oral appliance by assessing periodontal status and providing periodontal treatment.
- Provide patient instructions on how to use/clean an oral appliance and/or aligner (Table 4).
- Note changes in dentition and periodontium that may be caused by an oral appliance.
- Evaluate fit and integrity of an oral appliance.
- Professionally clean an oral appliance.
CONCLUSION
Through detection and treatment, oral health professionals can play a vital role in caring for patients with OSA. Screening questionnaires should be used to assess patients’ risk for OSA. If risk is shown, the appropriate referral should be made for further evaluation. When a patient has been diagnosed with OSA and the treatment plan calls for an oral appliance, the dental team (with a dentist who has been trained to fabricate an oral appliance) will assist the physician in the patient’s care. Through interprofessional collaboration, the dental team can help improve the quality of life for patients with OSA.
REFERENCES
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidmiol. 2013;177:1006–1014.
- Mayo Clinic. Obstructive Sleep Apnea: Symptoms and Causes. Available at: mayoclinic.org/diseases-conditions/obstructive-sleep-apnea/symptoms-causes/dxc-20205871. Accessed December 7, 2016.
- Epstein LJ, Kristo D, Strollo PJ, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–276.
- Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162:893–900.
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;238:1230–1235.
- Oksenberg A, Silverberg DS, Arons E, Radwan H. Positional vs nonpositional obstructive sleep apnea patients: antrhopomorphic, nocturnal polysomnographic, and multiple sleep latency test data. Chest. 1997;112:629–639.
- Peppard PE, Austin D, Brown RL. Association of alcohol consumption and sleep disordered breathing in men and women. J Clin Sleep Med. 2007;3:265–270.
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239.
- Wetter DW, Young TB, Bidwell TR, Badr MS, Palata M. Smoking as a risk factor for sleep-disordered breathing. Arch Intern Med. 1994;154:2219–2224.
- Blythe K. Patients with Type 2 Diabetes or Hypertension Must be Evaluated for Sleep Apnea. Available at: aasmnet.org/articles.aspx?id=3935. Accessed December 7, 2016.
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342;1378–1384.
- Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041.
- Ahmad NE, Sanders AE, Sheats R, Brame JL, Essick GK. Obstructive sleep apnea in association with periodontitis: a case-control study. J Dent Hyg. 2013;87:188–199.
- Sanders AE, Essick GK, Fillingim R, et al. Sleep apnea symptoms and risk of temporomandibular disorder: OPPERA Cohort. J Dent Res. 2013;92(7 Suppl):70S–77S.
- Cunali PA, Almeida FR, Santos CD, et al. Prevalence of temporomandibular disorders in obstructive sleep apnea patients referred for oral appliance therapy. J Orofacial Pain. 2009;23:339–344.
- Bender SD. Critical appraisal: sleep bruxism and sleep-disordered breathing. J Esthet Restor Dent. 2016;28:67–71.
- Carra MC, Huynh N, Lavigne G. Sleep bruxism: a comprehensive overview for the dental clinician interested in sleep medicine. Dent Clin North Am. 2012;56:387–413.
- Schellenberg JB, Maislin G, Schwab RJ. Physical findings and the risk for obstructive sleep apnea. The importance of oropharyngeal structures. Am J Respir Crit Care Med. 2000;162:740–748.
- Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proceedings of the American Thoracic Society. 2008;5:136–143.
- Chung F, Yegneswaran B, Liao P, et al. STOP Questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesioloy. 2008;108:812–821.
- Mallampati SR, Gatt SP, Gugino LD, et al. A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anesth Soc J. 1985;32:429–434.
- Nuckton TJ, Glidden DV, Browner WS, Claman DM. Physical examination: mallampati score as an independent predictor of obstructive sleep apnea. Sleep. 2006;29:903–908.
- Johns MW. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep. 1991;14:540–545.
- Mayo Clinic Staff. Polysomnography (Sleep Study). Available at: mayoclinic.org/tests-procedures/polysomnography/basics/definition/prc-20013229. Accessed December 7, 2016.
- Ferguson KA, Cartwright R, Rogers R, Schmidt-Nowara W. Oral appliances for snoring and obstructive sleep apnea: a review. Sleep. 2006;29:244–262.
- National Institutes of Health. CPAP. Available at: nhlbi.nih.gov/health/health-topics/topics/cpap. Accessed December 7, 2016.
- Camacho M, Certal V, Abdullatif J, et al. Myofunctional therapy to treat obstructive sleep apnea: a systematic review and meta-analysis. Sleep. 2015;38:669–675.
- Ramar K, Dort LC, Katz SG, et al. Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015. J Clin Sleep Med. 2015;11:773–827.
From Dimensions of Dental Hygiene. January 2017;15(1):38-41.



