Increase Your Knowledge of Restorative Materials
Dental hygienists routinely deal with prophylaxis of not only teeth, but a vast array of restorative materials. These restorations involve metallic, ceramic, polymeric, and composite materials that are part of the huge armamentarium representing old and new products employed over the past 50 years.

Dental hygienists routinely deal with prophylaxis of not only teeth, but a vast array of restorative materials. These restorations involve metallic, ceramic, polymeric, and composite materials that are part of the huge armamentarium representing old and new products employed over the past 50 years. More than 1,000 restorative products may be encountered, most of which are not specifically identified in a patient’s record. A dental hygienist needs to be able to recognize various restorative materials and employ the correct treatment protocol. The goals of this review are to summarize the key principles for safe finishing and polishing operations, consider the structure and properties of restorative materials that put them at risk, and identify precautions for dental hygiene procedures.
Photo Credit: ronstik / iStock / Getty Images Plus
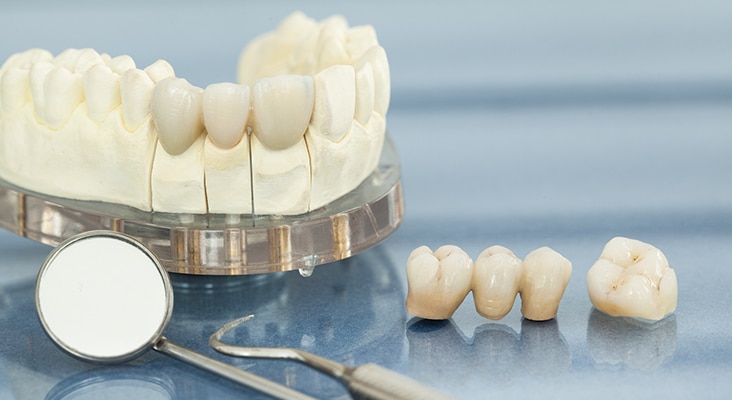
Types of Materials
Metals, ceramics, polymers, and composites are synthetic restorative materials. Metals include amalgam, removable partial denture frameworks, implants, gold, and other casting alloys. Ceramics may be porcelain, porcelain fused-to-metal, porcelain veneers, and high-strength ceramics. Polymers involve infiltrants and polymethyl methacrylate (PMMA) denture base materials. Composites encompass dental composites, glass ionomers, and temporaries. Management of these materials during a dental hygiene appointment requires some understanding of a material’s structure (arrangement, bonding, composition, defects) and properties (physical, chemical, mechanical, biological).
Photo Credit: seb_ra / iStock / Getty Images Plus
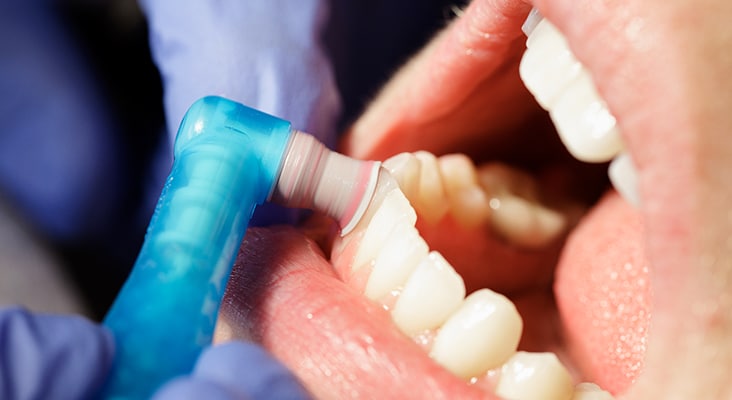
Protection During Prophylaxis
During a routine prophylaxis (plaque, calculus, and stain removal; surface smoothening) or prevention procedures (topical fluoride applications), a restorative material’s surface may be altered. Softer phases may be inadvertently or selectively removed. The dispersed phase is often chemically different and provides reinforcement properties. It may react differently to finishing and polishing. The best results are achieved by using polishing materials that are softer than both the dispersed and continuous phases of the restorative material.
Photo Credit: RichLegg / E+
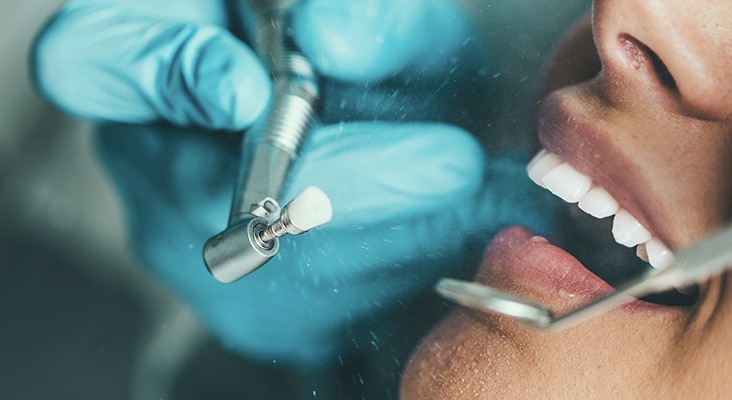
Hardness of Materials
Risks from wear or abrasion are relatively easy to rank in terms of a Mohs Hardness Scale. Hardness of any material is its mechanical resistance to plastic deformation. Mohs scale comparisons involve two materials being rubbed together to see which one is scratched by the other. This scale spans all material hardness, from the softest (talc = 1) to the hardest (diamond = 10). Hardness is 5–6 for enamel, 3–4 for dentin, and 2–3 for cementum. Polishing agents should be softer than enamel or any of the soft phases in a restorative material. While the primary consideration in polishing involves the hardness of materials, there are other factors, such as type of wear, duration of wear, applied pressure, and size of polishing particles. Larger particles produce greater wear. Smaller particles may erode softer phases. Dentifrices are designed to accomplish the same result as polishing procedures and are subject to the same conditions. Hardness ratios are typically used to summarize the relative likelihood of a potentially abrasive material to produce surface wear.
Photo Credit: microgen / iStock / Getty Images Plus
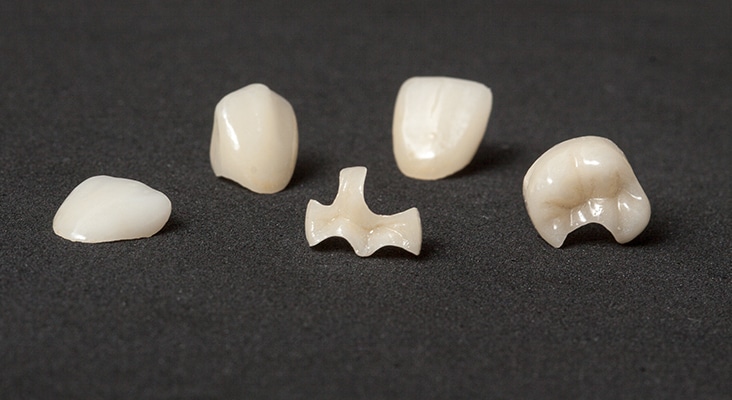
Caring for Tooth-Colored Materials
Tooth-colored materials (composites, glass ionomers, temporary, or provisional restorations). Without prior knowledge, it is generally difficult to identify a composite vs a glass ionomer restoration. Both are esthetic and have a continuous polymer phase with a dispersed silicate phase. Dental hygienists should be careful not to apply too much pressure during the polishing stage of the prophylaxis or the continuous polymer phase could slowly become abraded. Surface stains are easy to remove. Marginal stains associated with Class I, II, III, and V restorations, as well as veneers, involve discoloration that cannot be removed without damaging the restorative material. Do not aggressively polish at the margins. A variety of composites (macrofill, midifill, minifill, microfill, nanofill, and bi-hybrid or tri-hybrids, and glass ionomer materials) are available, but the various types are sufficiently similar that the same approach should apply.
Photo Credit: nospoon_pro / iStock / Getty Images Plus
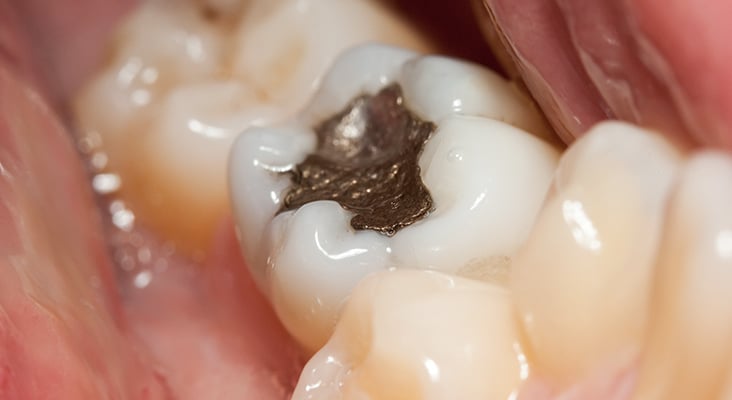
Amalgam
Tarnish or electrochemical corrosion products create a darkened or blackened appearance. Removing corrosion products produces a reflective metallic appearance that may be good for cleanliness but does not increase the material’s longevity. Inadvertent dry polishing of an amalgam and/or excessive pressure generates surface heat that easily melts the Ag2Hg3 reaction product (melting point = 127° C) within the continual phase—releasing and smearing Hg on the surface. The amalgam looks shiny because of its Hg-rich surface layer, but that smear layer is quickly lost over the next few days, exposing the patient and clinician to some Hg vapor during or post-procedure.
Photo Credit: icefront / iStock / Getty Images Plus

Titanium Implant Posts and Titanium Alloys
These materials are protected by a film of titanium dioxide (called a passivating film) that forms rapidly and clings tightly to the surface. It is so thin that it appears transparent and invisible. Scaling or aggressive polishing procedures remove this protective film. It will immediately reform if the surface is clean. Effective but not overly aggressive instrumentation strokes should be used, along with light polishing pressure and pumice and water. The same protective film may be disturbed by acidic reactions associated with some topical fluorides.
Photo Credit: Rost-9D / iStock / Getty Images Plus

Ceramic Materials
Ceramic is relatively resistant to degradation but surfaces can be partly dissolved by highly acidic intraoral solutions. Treatments with certain topical fluorides will also dissolve small bits of the surface. We have found that coating at-risk surfaces with a petroleum jelly film or other nonwater soluble agent is a simple way to provide temporary protection. As delivered, ceramic materials should have very smooth external surfaces. Any intraoral adjustments produce surface scratches that require smoothening with diamond finishing pastes (particle sizes approaching 0.1 µm). Ceramics have high hardness and therefore require zirconia or diamond polishing agents for smoothening. Ceramics are very susceptible to crack formation from stressed areas containing surface defects. If these defects are recognized, they should be removed with special small particle diamond polishing pastes. They cannot be removed with normal polishing materials during a dental hygiene procedure.
Photo Credit: okan akdeniz / iStock / Getty Images Plus
Dental Cements
Permanent restorations are attached with traditional acid-based cements, glass ionomer cements, or resin (composite) cements. The thickness of exposed cement at margins is typically 50 microns to 250 microns. These materials generally have lower hardness than restorative materials or tooth structure. Aggressive polishing may force abrasive material into the margin and potentially erode the cement. A technique of light pressure with prophy cup and prophy paste while polishing with swiping strokes across the margins is recommended.
Photo Credit: alex-mit / iStock / Getty Images Plus

Denture Base Materials
Most denture base polymer is PMMA with a hardness value similar to that of dentin. These materials are routinely cleaned with commercial denture base products and/or a soft toothbrush with soap and water. Abrasives in polishing materials or dentifrices will produce some surface scratching. Denture base material is commonly crosslinked by adding some difunctional monomer with the original methyl methacrylate monomers, which creates a more water-resistant material. Remember that a PMMA denture is prone to absorb water intraorally, and lose water when it is out of the mouth. Its mechanical properties vary as a function of water content. This is why dentures are stored in water when not being worn. Avoid procedures that would dry out the material.
Photo Credit: savcoco / iStock / Getty Images Plus

Gold Alloys
Alloys based on gold also corrode, but very slowly. Their surfaces are susceptible to electrochemical corrosion and over time may develop some surface pitting due to the presence of plaque. Therefore, gold restorations should be fully polished during recare visits. Rubber cup polishing with fine prophy paste is recommended. Any surface corrosion products that form are water soluble and, therefore, will not accumulate. Areas that are pitted can be polished, but will continue to slowly corrode if not kept clean.
Photo Credit: Muenz / iStock / Getty Images Plus

Bonding Systems
Bonding agents are extremely thin (< 5 µm) and only exposed at margins. Bonding agents are not at risk during routine prophylaxis and polishing operations. Do not attempt to remove stain that has crept into open margins of composites or veneers because of the possibility of damaging the margins. This situation is an esthetic failure and requires repair of margins.

