
Treatment Considerations For Heart Patients
Remaining up-to-date on the root causes and characteristics of cardiac arrhythmia and infective endocarditis helps to ensure the safe provision of dental care.
This course was published in the May 2015 issue and expires May 31, 2018. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Define cardiac arrhythmias and their effect on dental care.
- Discuss the etiology of infective endocarditis and its effects on the provision of dental treatment.
- Identify the patient populations that should receive antibiotic prophylaxis before dental treatment to prevent infective endocarditis.
With a prevalence of 82.6 million, cardiovascular disease (CVD) is the most common systemic disease in the United States.1 As such, dental hygienists can expect to treat a substantial number of patients with heart problems. Appropriate dental hygiene management requires an understanding of disease characteristics, pathophysiology, and treatment modifications to ensure optimal patient care. This article provides an overview of two common cardiovascular diseases: cardiac arrhythmia and infective endocarditis (IE).
CARDIAC ARRHYTHMIAS
More than 4 million Americans have some type of abnormality in their heart activity. The prevalence of cardiac arrhythmia increases with age.1 The most common form is atrial fibrillation, which affects more than 2.2 million individuals. Out of 1,000 older adults between the ages of 65 and 69, 11 have cardiac arrhythmias, while 60 out of 1,000 older adults age 80 and older experience this heart problem.1,2
Cardiac arrhythmias are a dysfunction of the heart rate or rhythm due to abnormalities in the generation and/or conduction of electrical impulses (Figure 1). When electrical impulses trigger cardiac contractions, the impulse follows a specific pathway through the heart and depolarization occurs. The natural pacemaker of the heart, the sinoatrial (SA) node is responsible for impulse formation and has a resting heart rate of 60 beats to 100 beats per minute. When an impulse leaves the SA node, it travels to the atrioventricular (AV) node, which is responsible for slowing down the conduction rate of impulses. Conduction rate is resumed as impulses travel through the ventricles via the bundle of HIS (heart muscle cells), which bifurcates into right and left bundle branches. Purkinje fibers extend from the bundle branches and carry the impulse to the myocardium of the ventricles. After depolarization, the ions return to their resting state (repolarization), allowing time for the SA node to recover. During this refractory period, depolarization cannot occur. Complete refractory—which results in conductivity block and partial refractory, causing a conductivity delay—can produce arrhythmias. Another possible cause of cardiac arrhythmias is the generation of an impulse by another part of the heart other than the SA node, which interrupts the heart’s normal activity.
There are several types of arrhythmias, including tachycardia (fast heart rate, greater than 100 beats per minute), bradycardia (slow heart rate, less than 60 beats per minute), atrial fibrillation, premature ventricular contractions, ventricular fibrillation, and heart block. Cardiac arrhythmias are treated with medications, pacemakers or implantable cardioverter-defibrillators (ICDs), radiofrequency catheter ablation, or surgery.3
DENTAL HYGIENE MANAGEMENT
During assessment, the dental hygienist should inquire about the type of arrhythmia, how it is being treated, medications taken, and whether the patient has an implantable device such as a pacemaker or ICD. It is important to determine what type of pacemaker the patient has and whether it is shielded from electromagnetic interference. Older pacemakers are not shielded and could be affected by dental equipment that generates electromagnetic fields such as ultrasonic devices. Newer pacemakers are shielded and should not disrupt the use of dental equipment.4
The use of ultrasonic scalers on patients with implantable devices, however, remains controversial. In an in vivo study by Maiorana et al,5 no interference was found between piezoelectric scalers and ICDs. This result supports the in vivo research conducted by Patel el al,6 which found no interference between ultrasonic scalers and pacemakers; the in vitro research by Gomez et al,7 which demonstrated no electromagnetic interference between a pacemaker and piezoelectric or magnetostrictive scalers; and the in vitro study by Brand et al,8 which noted no interference was present between pacemakers and ultrasonic scalers, even at a distance of 2.5 cm. Results from a 2015 clinical study suggest electrical devices used in dental offices have no interference with the pacing and sensing of contemporary ICDs or pacemakers.9 On the other hand, research by Roedig et al10 suggests that ultrasonic scalers cause electrical interference between cardiac pacemakers and ICDs. Using ultrasonic scalers on patients who have pacemakers and ICDs with protective coatings is generally considered safe. Consultation with the patient’s cardiologist may be prudent. More research is needed to further assess the effects of ultrasonic scalers on these devices.
Patients should be advised to always carry their device identification card that includes the device type, implant date, and their cardiologist’s name and phone number. If a patient does not have his or her device identification card and can’t recall the type and/or whether it is shielded, the patient’s cardiologist should be consulted.
Dental hygienists need to be aware of the oral side effects of medications taken by patients with arrhythmias. Patients with atrial fibrillation usually take an anticoagulant or antiplatelet medication to reduce the risk of blood clots and stroke; therefore, increased bleeding is a concern. Patients taking warfarin, an anticoagulant, should provide their international normalized ratio (INR)—a blood test that evaluates how well the blood clots. It is safe to treat patients with an INR less than 3.5.3 Employing local hemostatic measures, such as placing gelatin sponges or gauze pressure packs, is recommended. In addition, gingival overgrowth may be a clinical finding in patients taking calcium channel blockers.
Pain management as part of pre- and post-operative dental hygiene care is important for patients with arrhythmias to prevent cardiac crisis. For patients taking nonselective beta blockers, epinephrine should be used with caution as it may cause an increase in blood pressure; a maximum of 0.04 mg epinephrine (eg, two cartridges of anesthetic with 1:100,000 epinephrine) is recommended. Stress management protocols are also important to implement because anxiety can precipitate arrhythmias. Nitrous-oxide oxygen sedation may be appropriate for anxious patients.
Educating patients with cardiac arrhythmias on oral self-care techniques is important to promote not only oral health, but systemic health as well. Patients with cardiac arrhythmias have an increased risk of heart attack, cardiac arrest, and stroke.11 Therefore, promoting a healthy lifestyle is crucial. To manage risk factors, patients with an arrhythmia should control cholesterol levels, lose excess weight, eat a healthy diet, avoid tobacco, and engage in regular physical activity.11
INFECTIVE ENDOCARDITIS
Infective endocarditis, previously known as bacterial endocarditis, is an infection of the endocardial surface of the heart. The incidence varies based on the population surveyed, but a recent study conducted in the United States reported an incidence of 12.7 per 100,000 individuals per year.12 Although the incidence is low, infective endocarditis is a life-threatening disease with a mortality rate of approximately 40%.13 Infective endocarditis is more common in men than women and occurs most frequently in middle-aged to elderly populations.3 Patients with underlying heart conditions, such as mitral valve prolapse, aortic valve disease, congenital heart disease, and prosthetic valves, are at an increased risk for developing infective endocarditis.3,14 Several microorganisms are responsible for its development, but staphylococci and streptococci account for approximately 75% of cases.13 Although bacteria are the most common causative agent for infective endocarditis, fungi and viruses may also cause the disease.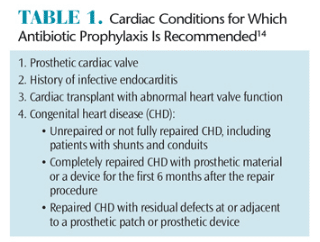
The pathophysiology of infective endocarditis involves a progression of events that generally originates with damage to the endothelium by a high volume of blood or mechanical injury. Small masses of sterile fibrin and platelets attach to the injured endothelium, resulting in nonbacterial thrombotic endocarditis. Initially, these masses do not contain any microorganisms. During transient bacteremia, however, bacteria can adhere to the small masses. The microorganisms stimulate the attachment of an additional protective vegetation layer of platelets and fibrin. Within the protective layer, the microorganisms multiply rapidly. Some vegetations detach and form embolisms, while others continually release bacteria into the bloodstream, causing constant bacteremia.
TREATMENT CONSIDERATION
Dental treatment can cause infective endocarditis in patients with underlying cardiac conditions. In an effort to reduce the risk of infective endocarditis, antibiotic prophylaxis has been recommended; however, the evidence to support this proactive treatment is lacking.3,15 Only a small number of infective endocarditis cases are caused by bacteremia induced by dental procedures and only a small number would be prevented, even if antibiotic prophylaxis was 100% effective.15 The American Heart Association still recommends antibiotic prophylaxis for all dental procedures that involve manipulation of gingival or periapical regions of the tooth or puncturing the oral mucosa for those patients at the highest risk for infective endocarditis (Table 1).3
Antibiotic prophylaxis is not necessary for local anesthetic injections in noninfected tissue, exposing dental radiographs, placement of removable dentures, shedding of primary teeth, or trauma to the lips or oral mucosa.15 Based on the American Heart Association guidelines, which were updated in 2007, patients with mitral valve prolapse and rheumatic heart disease no longer need antibiotic prophylaxis prior to dental treatment. This revision should be discussed with patients so they understand the reasons for this change. Including the patient’s physician during this discussion may be helpful.
A thorough review of the patient’s medical history is necessary to identify those susceptible to infective endocarditis. If any doubt exists, the clinician should consult the patient’s physician. Prior to recommending antibiotics, patients should be asked about current medications and allergies so an appropriate antibiotic can be selected (Table 2). If the patient is taking an antibiotic that is generally recommended for antibiotic prophylaxis prior to dental treatment, an antibiotic from a different class should be chosen rather than increasing the dosage of the current antibiotic. For example, if a patient was on a long-term penicillin regimen, oral streptococci may be relatively resistant to that class; therefore, clindamycin, azithromycin, or clarithromycin should be selected for infective endocarditis prophylaxis prior to dental procedures. It is best to wait 10 days after the patient’s antibiotic therapy is completed to perform the dental procedure in order to allow the oral microflora to regenerate.15
Poor oral hygiene and gingivitis are risk factors for infective endocarditis.15–17 The risk of bacteremia from a dental procedure is low compared to the magnitude of bacteria resulting from daily activities such as chewing, brushing, and flossing.3,15,18,19 As such, patients at increased risk for infective endocarditis should be educated on the importance of effective oral self-care to reduce the frequency of bacteremia.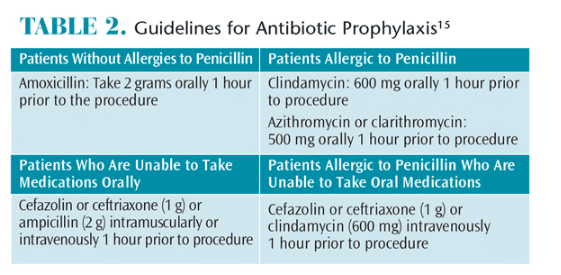
Patients with periodontal diseases should be educated on the possible systemic effects of the disease and the importance of eliminating active infection. Pretreatment chlorhexidine mouthrinses are recommended prior to all procedures to reduce the presence of oral bacteria.4 Depending on the patient’s ability to tolerate long appointments, the dental team should try to complete multiple procedures in one appointment to reduce the risk of the patient developing resistant bacteria. If treatment cannot be completed in one appointment, the patient’s dental appointments should be scheduled at least 7 days—but preferably 10 days to 14 days—apart, and a different antibiotic regimen should be recommended.4 Furthermore, research by Chen et al20 indicates patients who receive dental scaling at least one time per year experienced a 33% reduction in their risk of infective endocarditis. Therefore, frequent and regular recare should be emphasized to this patient population.4,20
CONCLUSION
Cardiac arrhythmias and infective endocarditis are common cardiovascular diseases that affect a wide spectrum of Americans. Dental hygienists must develop an understanding of disease characteristics, antibiotic prophylaxis guidelines, stress and pain management protocols, and medication side effects to ensure appropriate treatment modifications are implemented so these patients can receive oral health care services safely.
REFERENCES
- Tang DH, Gilligan AM, Romero K. Economic burden and disparities in healthcare resource use among adult patients with cardiac arrhythmia. Appl Health Econ Health Policy. 2014;12:59–71.
- Rich MW. Epidemiology of atrial fibrillation. J Interv Card Electrophysiol. 2009;25:3–8.
- Little JW, Falace DA, Miller CS, Rhodus NL. Dental Management of the Medically Compromised Patient. 8th ed. St Louis: Elsevier; 2013.
- Newman M, Takei H, Klokkevold P, Carranza F, eds. Carranza’s Clinical Periodontology. 12th ed. St. Louis: Elsevier; 2015.
- Maiorana C, Grossi G, Garramone R, Manfredini R, Santoro F. Do ultrasonic dental scalers interfere with implantable cardioverter defibrillators? An in vivo investigation. J Dent. 2013;41:95–959.
- Patel D, Glick M, Lessard E, Zaim S. Absence of in vivo effects of dental instruments on pacemaker function. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:430.
- Gomez G, Jara F, Sánchez B, Roig M, Duran-Sindreu F. Effects of piezoelectric units on pacemaker function: an in vitrostudy. J Endod. 2013;39:1296–1299.
- Brand H, van der Hoeff EV, Schrama TA, Entjes ML, van Nieuw AA. Electromagnetic interference of electrical dental equipment with cardiac pacemakers. Nederlands Tijdschrift Voor Tandheelkunde. 2007;114:373–376.
- Elayi CS, Lusher S, Meeks Nyquist JL, Yousef D, Morales GX, Miller CS. Interference between dental electrical devices and pacemakers or defibrillators. J Am Dent Assoc. 2015;146:121–128.
- Roedig JJ, Shah J, Elayi CS, Miller CS. Interference of cardiac pacemaker and implantable cardioverter-defibrillator activity during electronic dental device use. J Am Dent Assoc. 2010;141:521–526.
- American Heart Association. Prevention and Treatment of Arrhythmia. Available at: heart.org/ HEARTORG/Conditions/Arrhythmia/PreventionTreatmentofArrhythmia/Prevention-Treatment-of-Arrhythmia_UCM_002026_Article.jsp. Accessed April 23, 2015.
- Bor D, Woolhandler S, Nardin R, Brusch J, Himmelstein D. Infective endocarditis in the US, 1998–2009: A Nationwide Study. Plos ONE. 2013;8:1–8.
- Bashore TM, Cabell C, Fowler V. Update on infective endocarditis. Curr Probl Cardiol. 2006;31:274–352.
- American Heart Association. Infective Endocarditis. Available at: heart.org/HEARTORG/Conditions/ CongenitalHeartDefects/TheImpactofCongenitalHeartDefects/InfectiveEndocarditis_UCM_307108_Article.jsp. Accessed April 23, 2015.
- Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: Guidelines from the American Heart Association: A Guideline from the American Heart Association Rheumatic Fever, Endocarditis and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. J Am Dent Assoc. 2007;138:739–760.
- Lockhart P, Brennan M, Sasser H, et al. Poor oral hygiene as a risk factor for infective endocarditis-related bacteremia. J Am Dent Assoc. 2009;140:1238–1244.
- Tomás I, Diz P, Tobías A, Scully C, Donos N. Periodontal health status and bacteraemia from daily oral activities: systematic review/meta-analysis. J Clin Periodontol. 2012;39:213–228.
- Seymour RA. Dental treatment, antibiotic cover and infective endocarditis: a major rethink. Dental Update. 2008;35:366–370.
- Crasta K, Daly C, Mitchell D, Curtis B, Stewart D, Heitz-Mayfield L. Bacteraemia due to dental flossing. J Clin Periodontol. 2009;36:323–332.
- Chen S, Liu C, Chiang C, et al. Dental scaling and risk reduction in infective endocarditis: a nationwide population-based case-control study. Can J Cardiol. 2013;29:429–433.
From Dimensions of Dental Hygiene. May 2015;13(5):49–52.



