
The Signs and Symptoms of Hereditary Hemorrhagic Telangiectasia
Though this genetic disorder is relatively rare, the first manifestations often appear in the oral cavity, so dental professionals need to be prepared to recognize them.
This course was published in the November 2013 issue and expires November 2016. The author has no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Detail the prevalence of hereditary hemorrhagic telangiectasia (HHT).
- Describe the etiology of HHT.
- Identify the presentations of HHT.
- Discuss the role of the dental professional in detecting and addressing HHT.
HHT from hemophilia.1 Underreported for many years, the true prevalence of HHT is unknown. Estimates based on the literature vary and are dependent on the region assessed—with a median estimated prevalence of one to two cases per 10,000 people. HHT has a wide ethnic and geographic distribution throughout Europe and North America, but is reportedly rare in people of African, Asian, and Arabic descent.1–3 Shioya et al2 have estimated the prevalence of HHT to be approximately one in 2,351. Haitjema et al3 and Westermann et al4 estimate HHT prevalence to be around one in 100,000. The HHT Foundation asserts that approximately 1.4 million people worldwide are affected. The HHT Foundation and the United States Centers for Disease Control and Prevention (CDC) co-sponsored a conference entitled “HHT Health Initiatives for the 21st Century.” The conference members ascertained that approximately 90% of individuals with HHT remain undiagnosed—putting them at risk for sudden rupture of the blood vessels in major organs of the body.5,6
ETIOLOGY
The genetic aspect of HHT has been studied extensively for the past several years. The condition is part of a family of disorders caused by mutations in multiple genes. An abnormal gene on either chromosome 9 (endoglin) or 12 (active-like kinase, or ALK-1) causes most cases of HHT.5 Both genes are involved in the formation and repair of blood vessels. Genetic linkages to HHT have found mutations to chromosome 9q33-q34 in some families and to chromosome 12q in others. This might explain the vast differences in clinical appearances among individuals affected by HHT. Currently, HHT is divided into two subgroups: HHT type 1 (HHT 1) and HHT type 2 (HHT 2). Some references state, however, that there is a possible third type. The chromosomal involvement determines the HHT type. In HHT type 1, mutations at chromosome 9 alter the coding sequence of endoglin. In HHT type 2, mutations at chromosome 12 alter the coding sequence of ALK-1.6
Genetic mapping completed over the past few years has found that HHT is linked to loci on at least three different chromosomes. Endoglin, the most abundant transforming growth factor beta (TGF-?) binding protein found on epithelial cells, has been identified as the HHT gene mapping to locus 9q3, and is presently referred to as the gene for HHT 1. Thus far, at least 16 novel mutations of the endoglin gene have been discovered in 17 families. Proposed mechanisms by which mutations to the endoglin gene might cause HHT 1 include a dominant negative effect, a two-hit model, and haploinsufficiency (when an individual with a gene mutation has a single copy of the normal gene and, thus, is incapable of generating enough protein to enable normal function). The most recent data seem to support haploinsufficiency as the most likely cause.7
Mutations in the gene for ALK-1 have been mapped to locus 12q and are referred to as the gene for HHT 2. ALK-1 is a type I serin-theronine kinase receptor that can bind TGF-? in the presence of its type II receptor and is expressed predominantly on endothelial cells. Thus far, at least 12 novel mutations of the ALK-1 gene have been discovered in 12 families. Each of the 12 mutations has affected the cytoplasmic kinase region, suggesting that the resulting protein has decreased kinase activity. As with the endoglin gene, there is evidence to support the dominant negative, two-hit, and haploinsufficiency mechanisms. Preliminary data suggest a third locus for HHT at 3q22, where the TGF-? II receptor gene is located.8
PRESENTATION
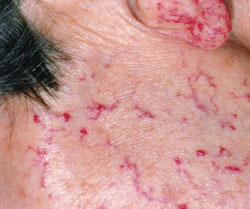
HHT is characterized by several telangiectasias throughout the body. These vascular malformations occur when venules and arterioles become convoluted, leak vascular fluid into surrounding tissues, and eventually completely bypass capillary beds.7 The telangiectasia is the characteristic lesion of HHT (Figure 2). It arises from a dilated post capillary venule that enlarges and fuses with an arteriole, bypassing the capillary system and resulting in an arteriovenous communication.
The lesion can present as multiple diffuse telangiectasias or as discrete arteriovenous malformations. A person with HHT has a tendency to form blood vessels that lack the capillaries between an artery and vein. This means that arterial blood under high pressure flows directly into the vein without first having to squeeze through the very small capillaries. The area of connection between an artery and a vein tends to be fragile, and can rupture, leading to bleeding. Clinically, these abnormalities manifest in several areas of the body.
Because of the many clinical signs of HHT that appear in the oral cavity, head, and neck, dental professionals hold a vital position in the health care strata to detect these cases. HHT manifests in the oral cavity as red spots approximately 1 mm to 3 mm in diameter that appear on the tongue, lips, palate, or gingival tissue. Manipulation of these lesions during dental treatment—such as during prophylaxis, polishing, tissue retraction, tooth brushing, and flossing—can cause them to bleed.
Epistaxis (nosebleed) is the most common—and typically the earliest—manifestation of HHT, occurring in up to 95% of affected patients. Bleeding may be mild or severe, can progress with age, and often results in chronic anemia. Mucocutaneous telangiectasias (red spots from direct junction of venule and arteriole making the dilatation) usually involve the buccal mucosa, face, extremities, or nasal mucosa. These external signs of the disease may not manifest until affected individuals are in their 20s and 30s.9
Patients may experience significant effects on their quality of life or need to be hospitalized because of epistaxis. Patients affected by these occasional or daily recurrent nosebleeds only require iron supplements. Others may need blood transfusions, nasal packing, or more extensive treatments. Temporary relief is often accomplished with electrocauterization and hormonal therapy, but long-term success can be provided by laser cauterization or septal dermoplasty involving skin grafts in the inner nose.
Oral health professionals should be aware that patients with chronic nosebleeds reported in health screening questionnaires could have HHT.10 Dentists and dental hygienists who observe telangiectasias of the oral cavity or skin in patients with a history of nosebleeds should suspect that they may have this genetic condition. A definitive diagnosis is not achieved, however, until both of these criteria are met in addition to the presence of AVM and a first-degree relative with the same symptoms.10
HHT has traditionally been diagnosed on the basis of its clinical features, known as the Curaçao criteria. The definitive clinical diagnosis of HHT is based on the presence of at least three of four main clinical features: epistaxis (usually present since childhood); cutaneous or mucosal (oral or intranasal) telangiectasias; visceral involvement (lung, central nervous system, GI tract, or liver); and a family history of HHT (Table 1). Additionally, within HHT families, a firm diagnosis of HHT can be made on the basis of two separate visceral manifestations.
The GI tract, liver, lungs, and neurological system can all be affected by HHT. The primary GI presentation of HHT is painless bleeding from AVMs and telangiectasias in the upper and lower bowel.11 Unlike epistaxis, GI bleeding is typically delayed until individuals reach their 50s and 60s,1,12 and occurs in approximately 40% of patients with HHT. The presence of AVMs in the GI tract can result in a number of complications, including hemorrhage, iron deficiency, anemia, progressive liver disease, and high output heart failure.12 Iron supplementation and transfusion may be required for supportive treatment.10 AVMs in the liver occur in about 30% of patients with HHT and are usually asymptomatic. High output heart failure, portal hypertension, and biliary disease are the AVMs that most commonly present with symptoms.
Treatment of hepatic AVMs has changed. Because embolizations (blocking or occluding blood flow to a blood vessel) have caused fatal hepatic necrosis, a moratorium has been placed on this treatment unless supported by a liver transplantation program.13
According to Begbie et al,13 pulmonary AVMs (PAVMs) are thin-walled, abnormal vessels that replace normal capillaries between the pulmonary arterial (artery) and venous (vein) circulations. Catastrophic embolic cerebral events (brain abscess and embolic stroke) and transient ischemic attack (TIA) can occur in patients, regardless of the degree of respiratory symptoms. Up to 50% of patients with pulmonary AVMs are asymptomatic before neurologic effects appear, such as brain abscess or stroke.10 These symptoms are often the first manifestations of pulmonary AVMs, or even HHT itself.1
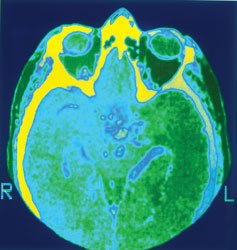
Regular monitoring with computed tomography (CT) scans is recommended, and embolization of AVMs is the treatment of choice. The administration of prophylactic antibiotics may be indicated at the time of dental and surgical procedures to reduce the risk of brain abscess.13 Neurological manifestations of HHT can include brain abscess (Figure 3), TIA, stroke, spinal and cerebral AVMs, seizures, intracranial hemorrhage, and migraine headaches, which are the most common symptom. These conditions can arise from primary vascular lesions in the central nervous system, or they may be secondary to pulmonary vascular lesions.10
Patients with HHT are 1,000 times more likely to develop a brain abscess than the general population.10 Symptoms related to an abscess vary depending on the size and location of the affected area. Routine use of CT scans and magnetic resonance imaging (MRI) has made early detection of these lesions possible. Treatments for the abscess may include surgical drainage, monitoring with CT scans and MRI, and intravenous antibiotics. With improvements in imaging, surgical techniques, and antibiotic efficacy, the morbidity and mortality rates associated with brain abscess should continue to decline.10
DENTAL PRECAUTIONS
Dental professionals are vital in referring patients who may have HHT for diagnosis and treatment due to the syndrome’s oral manifestations. According to Shovlin et al,14 dental bacteria can cause endocarditis or other infections following dental bacteremias induced by invasive dental procedures. PAVMs, silent vascular abnormalities in the pulmonary circulation that result in a right-to-left shunt (from pulmonary artery to pulmonary vein, bypassing the pulmonary capillary bed), generally occur in association with HHT. Patients with HHT and PAVMS are also at risk during invasive dental procedures because the bacteria introduced into the bloodstream from the gingiva can lead to brain abscess.13 Shovlin et al14 found that the major organisms isolated from brain abscess aspirates were the same anaerobic bacteria commonly found in the oral cavities of individuals with periodontal infections.
Typically, antibiotic prophylaxis has been recommended for individuals with HHT and PAVMs due to the bacterial risk presented during dental treatment. The National Institute for Health and Clinical Excellence’s (NICE) most recent guidelines, however, no longer recommend antibiotic prophylaxis for patients with structural heart disease at risk of infective endocarditis.14 The AHA has also reached similar conclusions for premedication in adults and children with structural heart disease who are at risk for infective endocarditis.15 It is possible that the NICE and AHA committees did not consider people with HHT and PAVM when making these recommendations.14 Presently, there are no clear premedication guidelines for patients with HHT and PAVMS, except for those at greatest risk of adverse outcomes resulting from endocarditis.14
Upon determination that a patient with HHT and PAVMs needs antibiotic prophylaxis, he or she should receive amoxicillin. This is because oral bacteria, particularly the viridans group of streptococci associated with endocarditis, are most sensitive to drugs in the penicillin family.14 For individuals with PAVMs and HHT who are allergic to penicillin, clindamycin can be used. Patients with HHT should only take nonsteroidal antiinflammatory analgesics, such as ibuprofen, for a short period of time due to the risk of GI bleeding and effects on platelet function.14 Anticoagulants, such as aspirin, should be avoided due to the risk of epistaxis.5
CASE REPORTS
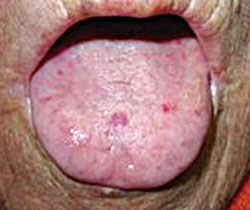
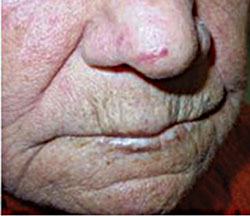
Dental practitioners play a valuable part in the diagnosis of HHT because the first signs often appear in the oral mucosa. According to a case report from da Silva Santos et al,16 a 74-year-old woman’s dentist recognized clinical symptoms of HHT. The patient’s medical history was extensive including: congestive heart failure, chronic renal failure, hypertension, hypothyroidism, and rheumatism. Due to vascular disorders, her lower right extremity had been amputated. She had a family history of telangiectasias and epistaxis. Extraoral and intraoral examinations were completed, including panoramic and periapical radiographs, in order to develop a dental treatment plan. Periodontitis and caries were both present. Telangiectasias were noted, especially on the oral mucosa of the tongue (Figure 4), hard palate, and vermillion of the lip.16 They were also present on the face (Figure 5) and upper extremities.16 The patient’s laboratory tests showed significant changes in red blood cell count, but no changes in coagulation.
Because the clinical signs and symptoms seemed to suggest HHT, she was referred to an internal medicine specialist, who confirmed the diagnosis. Before dental treatment was performed, prophylactic amoxicillin (500 mg) was administered every 8 hours (beginning 12 hours before treatment and extending for 7 days) to prevent the risk of cerebral abscess or pulmonary infections. Other precautions, such as using a vertical dental chair, were taken during treatment to help prevent lung and nasal bleeding. Additionally, the patient’s blood pressure was closely monitored during treatment.16
According to el-Houcheimi et al’s17 case report, a 40-year-old man was admitted to the hospital with chest pain, upper abdominal pain, and a fever. Routine tests came back normal. His fever and pain departed spontaneously, and he was discharged 3 days later. Two days later, he was admitted to another hospital with a severe headache and left-sided weakness. Another CT scan of the brain revealed a cerebral abscess. Numerous skin and facial telangiectasia were present. The patient had a family history of HHT, including his mother, uncle, and sister. Two weeks prior to the symptoms occurring, the patient had been to the dentist where he was diagnosed with periodontitis and suppuration was drained from an infected tooth. There was no mention of premedication prior to dental treatment.17
It is unfortunate that the dental professionals in this case did not recognize the signs of HHT. Careful review of medical history, examination of external structures (part of head and neck physical exam), patient oral risk assessment, and critical thinking could have helped the team members note the potential for this disorder.17
CONCLUSION
HHT is a vascular condition with a myriad of manifestations. It is characterized by the presence of multiple AVMs that lack intervening capillaries, resulting in the direct connection between arteries and veins. Epistaxis and mucocutaneous telangiectasis are the most common clinical signs. It is estimated that approximately 90% of individuals with HHT are not yet diagnosed and may be at risk for sudden rupture of the blood vessels in major organs.6 Strokes, seizures, intracranial hemorrhage, migraine headaches, portal hypertension, biliary disease, GI bleeding, and anemia are just some examples of HHT’s signs and symptoms. Patients with HHT are also at increased risk for brain abscess.18
Dental professionals play an important role in recognizing HHT because the first symptoms usually appear in the oral mucosa. Unfortunately, most dental curricula do not include information regarding HHT. Because of this, many dental professionals are unaware of its oral manifestations, the possible need for prophylactic antibiotics before invasive dental procedures, and that common anti-inflammatory medications should not be prescribed. Little research has been completed in the US; thus, most of the available data appear in international journals, which may not be frequently reviewed by American oral health professionals. No clear recommendations currently exist for prophylactic antibiotic treatment for patients with HHT prior to dental procedures. A standardized protocol should be established due to the correlation between invasive dental procedures and life-threatening cerebral abscess.
To improve the standard of care for patients with HHT, further research by the dental and medical communities is indicated and greater awareness of the disease is needed.
ACKNOWLEDGEMENT
The author would like to thank Janice Cox, MSLS, MA, the head librarian at Indiana University School of Dentistry, for her contribution to this literature review.
REFERENCES
- Guttmacher AE, Marchuck DA ,White RI Jr.Hereditary hemorrhagic telangiectasia. N Engl JMed. 1995;333:918–924.
- Shioya T, Hashimoto M, Koizumi A,Kawamura M, Miura M. Hereditary hemorrhagictelangiectasia (HHT) in Akita Prefecture, Japan.Intern Med. 2000;39:675–676.
- Haitjema T, Westermann CJ, Overtoom TT,et al. Hereditary hemorrhagic telangiectasia(Osler-Weber-Rendu Disease): new insights inpathogenesis, complications, and treatment.Arch Intern Med.1996;156:714–719.
- Westermann CJ, Rosina AF, De Vries V,de Coteau PA. The prevalence andmanifestations of hereditary hemorrhagictelangiectasia in the Afro-Caribbean populationof the Netherlands Antilles: a family screening.Am J Med Genet A. 2002;116A:324–328.
- HHT Foundation International Inc (Osler-Weber-Rendu Syndrome). Available at: hht.org.Accessed October 24, 2013.
- Centers for Disease Control and Prevention.Health Initiatives for the 21st Century: ExecutiveSummary, HHT Conference, March 5-6, 2008.Available at: www.hht.org/docs/CDC_Final_HHT_Report_June09.pdf. Accessed October 24, 2013.
- Gossage JR, Kanj F. Pulmonary arteriovenousmalformations. A state of the art review. Am JRespir Crit Care Med.1998;158:643–661.
- Kjeldsen AD, Vase P, Green A. Hereditaryhaemorrhagic telangiectasia: a populationbasedstudy of prevalence and mortality inDanish patients. J Intern Med. 1999;245:31–39.
- Ragsdale JA. Hereditary hemorrhagictelangiectasia: from epistaxis to life-threateningGI bleeding. Gastroenterol Nurs. 2007;30:293–300.
- Sell B, Evens J, Horn D. Brain abscess andhereditary hemorrhagic telangiectasia. SouthMed J. 2008;101:618–625.
- Christensen GJ. Nosebleeds may meansomething much more serious: an introductionto HHT. J Am Dent Assoc.1998;129:635–637.
- Peery WH. Clinical spectrum of hereditaryhemorrhagic telangiectasia (Osler-Weber-Rendudisease). Am J Med.1987;82:989–997.
- Begbie ME, Wallace GM, Shovlin CL.Hereditary haemorrhagic telangiectasia (Osler-Weber-Rendu syndrome): a view from the 21stcentury. Postgrad Med. 2003;79:18–24.
- Shovlin C, Bamford K, Wray D. Post-NICE2008: Antibiotic prophylaxis prior to dentalprocedures for patients with pulmonaryarteriovenous malformations (PAVMs) andhereditary haemorrhagic telangiectasia. Br Dent J.2008;205:531–533.
- American Heart Association. InfectiveEndocarditis. Available at: www.heart.org/HEARTORG/Conditions/CongenitalHeartDefects/TheImpactofCongenitalHeartDefects/Infective- Endocarditis_UCM_307108_Article.jsp. AccessedOctober 24, 2013.
- da Silva Santos PS, Fernandes KS,Magalhães MH. Osler-Weber-Rendu syndrome–dental implications. J Can Dent Assoc.2009;75:527–530.
- el-Houcheimi I, Hardwidge C, Walter P,Jalauddin M. Brain abscess and hereditaryhaemorrhagic telangiectasia: a report of threecases. Brit J Neurosurg.1998;12:15–17.
- Flint SR, Keith O, Scully C. Hereditaryhemorrhagic telangiectasia: family study andreview. Oral Surg Oral Med OralPathol.1988;66:440–444.
From Dimensions of Dental Hygiene. November 2013;11(11):55–58,61.



