 STEVE GSCHMEISSNER/SCIENCE PHOTO LIBRARY
STEVE GSCHMEISSNER/SCIENCE PHOTO LIBRARY
The Root of the Problem
To effectively manage dentinal hypersensitivity, the causative factors must first be identified.
Tooth pain and sensitivity are frequent patient complaints. The oral health care team often collaborates on recommended treatment options for patients, with some offering relief within a couple of hours or days. However, this respite can be short-lived if the site of sensitivity is not properly addressed. Therefore, it is critical to identify the causative factors behind sensitivity to effectively manage the problem.1
Dentinal hypersensitivity is characterized by a short, sharp pain arising from exposed dentin that occurs in response to stimuli, typically thermal (hot or cold), evaporative, tactile, osmotic, or chemical that cannot be ascribed to any other dental defect or pathology.2–4 Dentinal hypersensitivity has been referred to as one of the most painful and chronic dental conditions, with reported prevalence between 4% and 57% in the general population, and 60% to 98% of periodontal patients.5–8 The incidence of sensitivity is slightly higher among women than men. While dentinal hypersensitivity can affect patients of all ages, it is most common among those age 20 to 50, with a peak occurring between age 30 and 40.9 Canines and premolars of both arches are the most frequently affected, with the buccal aspect of the cervical area serving as the most frequently affected site.10
ETIOLOGY
Dentin is covered and protected by hard tissues, including enamel and cementum. A vital tissue, dentin is naturally sensitive because of the extension of odontoblasts, which contain pain-sensing nerve fibers, and the existence of the dentin-pulp complex.11,12 Dentin consists of numerous dentinal tubules that traverse from the pulp of the tooth to outer dentinal fibers.1 Three types of sensory fibers exist within dentin: A-delta, A-beta, and C-fibers.
A-deltas are small myelinated fibers that evoke short, sharp pain responses and are thought to be responsible for dentinal hypersensitivity. 1 A-beta fibers are susceptible to the same types of stimuli but respond more sensitively to electrical stimulation. In contrast to the A-delta and A-beta fibers, stimulation of unmyelinated C-fibers results in a more aching pain response, usually associated with pulpal pain.1
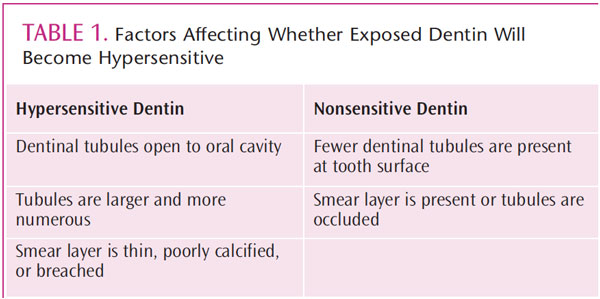
The hydrodynamic theory is the most widely accepted explanation of dentinal hypersensitivity. First described by Brannstorm,1,5,13 this hypothesis proposes that hypersensitivity is caused by pressure on the nerves, resulting from changes in fluid found within the dentinal tubules (Figure 1). The changes in the fluid are caused by a stimulus (thermal, tactile, or chemical). Approximately 75% of patients with dentinal hypersensitivity complain of pain when a cold stimulus is present.9 Although exposed dentin is generally associated with hypersensitivity, it is not always the cause.2 Hypersensitive dentin has the following characteristics: ends of dentinal tubules are open to the oral cavity, tubules are larger and more numerous, and smear layer is thin, poorly calcified, or breached.1 In contrast, nonsensitive dentin has fewer dentinal tubules at the tooth surface, and either a smear layer is present or tubules are occluded by mineral compounds (Table 1).1
IDENTIFYING THE SOURCE
The first thing many patients share with their dental hygienist is their concern about sensitivity. In most situations, patients are experiencing on and off moments of discomfort. For example, the area of sensitivity could be exacerbated by a particular stimulus. Therefore, once the complaint of sensitivity is established, the dental professional needs to investigate the cause. As part of the assessment, the dental professional should ask how long the sensitivity lasts, when it was first noticed, and what stimulus causes the reaction. Before a diagnosis is made, however, a thorough clinical history and radiographic examination should be performed. In addition, other conditions must be ruled out, including occlusal trauma, caries, defective restorations, fractured or cracked teeth, and potential reversible or irreversible pulpal pathology.10,14
Exposed root surfaces due to gingival recession are a major predisposing factor to dentinal hypersensitivity.15 Common causes of gingival recession include frenal involvement, inadequate attached gingiva, loss of attachment during restorative procedures, acute or chronic trauma, excessive brushing or flossing, and poor oral hygiene.1,2,5 Exposed root surfaces may also be the result of certain periodontal surgical procedures. Toothbrush abrasion is common among young people, often due to the use of excessive force while brushing and/or using a medium- to hard-bristle toothbrush. Erosion is caused by consuming acidic foods or drinks and/or persistent vomiting. Patients who have frequent acidic exposures should avoid brushing right after the exposure.5
Tooth whitening can also cause sensitivity, and patients should be informed about the short-term sensitivity that may result.5 Many oral conditions exhibit symptoms similar to dentinal hypersensitivity, therefore, a differential diagnosis is necessary.
MANAGING DENTINAL HYPERSENSITIVITY
There are two major groups of products used to manage dentinal hypersensitivity: those that block and occlude dentinal tubules, and those that interfere with the transmission of neural impulses.1,5,13,16 Depending on the severity of the sensitivity, two treatment options are available: at-home desensitizing agents for mild sensitivity, and professional treatment for moderate to severe sensitivity.1,5
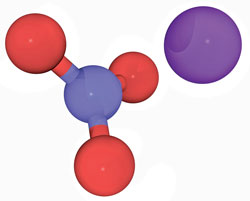
Self-applied desensitizing agents are recommended for patients with mild sensitivity. Patients need to understand that continuous use over an extended timeframe is required for efficacy with these products.1 Self-applied agents are cost-effective, noninvasive, and simple to use. Potassium nitrate is the most common desensitizing agent used in over-the-counter dentifrices (Figure 2).1 Most products contain 5% potassium nitrate in conjunction with sodium or monofluorophosphate fluoride and they reduce symptoms within 2 weeks of daily use.1 Other self-applied desensitizing agents are available in gels, mouthrinses, pastes, and dentifrices with various fluoride compounds, such as sodium fluoride, sodium silicofluoride, and stannous fluoride, as well as calcium phosphate technologies, as active ingredients.1
Although self-applied desensitizing agents are adequate for mild cases of sensitivity, professionally-applied agents are highly recommended for moderate to severe cases.1 Fluoride varnish is an effective chairside treatment that occludes the dentin tubules through the application of calcium fluoride to the tooth surface. Calcium phosphate technologies, including amorphous calcium phosphate (ACP), casein phosphopeptide-amorphous calcium phosphate (Recaldent®), calcium sodium phosphosilicate (NovaMin®), and tricalcium phosphate (TCP), are also used to address dentinal hypersensitivity. All of these technologies increase the availability of calcium and phosphate ions in saliva in order to promote tooth remineralization, which may help reduce dentinal hypersensitivity. The combination of arginine and calcium carbonate is another sensitivity therapy that is designed to plug and seal open dentinal tubules. Available in a paste, this in-office therapy is applied with a rotary cup. Some of these options are also available for at-home use, including a gel with ACP, a prescription paste with Recaldent, and prescription dentifrices with NovaMin and TCP, respectively.17,18
In severe cases, the loss of cervical tooth structure may require restoration with glass ionomer and/or composite resin materials to control hypersensitivity. Three other treatment options for severe cases include iontophoresis, lasers, and periodontal plastic surgery.1 Iontophoresis involves the delivery of sodium fluoride by passing an electrical current through the cervical dentin. Laser therapy is a relatively quick procedure that involves one treatment that drastically reduces or eliminates sensitivity by sealing the dentinal tubules.1 Periodontal plastic surgery used to treat gingival recession generally involves removing connective tissue (usually from the palate) and placing it on top of exposed root.1 This treatment may increase clinical attachment, as well as reduce dentinal sensitivity after 6 months.17
RECOMMENDATIONS FOR DENTAL HYGIENISTS
After the cause of hypersensitivity and treatment options are established, patients should be educated about behaviors that could exacerbate their symptoms. Patients with dentinal hypersensitivity should avoid the use of medium- to hard-bristle toothbrushes, over brushing or using too much pressure, excessive flossing or improper interdental cleaning, and irritating the gingival margin.5 When treating patients with dentinal hypersensitivity, dental hygienists should also avoid over instrumenting root surfaces during scaling and root planing, particularly in the cervical area, and over polishing exposed dentin during stain removal.5
CONCLUSION
Completing a comprehensive medical and dental history is the first step, followed by a radiographic examination, in determining the cause of dentinal hypersensitivity. Finding the causative factor and discovering a differential diagnosis are imperative to providing an effective treatment plan for the patient. It is important for dental hygienists to stay up-to-date about the various treatment options for patients with mild to severe dentinal hypersensitivity. While providing patients with relief from their sensitivity is the main goal, educating them about how to best manage and prevent symptoms is equally important.
REFERENCES
-
- Darby M, Walsh M. Dental Hygiene Theory and Practice. 3rd ed. St. Louis: Saunders Elsevier; 2010.
- Miglani S, Aggarwal V, Ahuja B. Dentin hypersensitivity: recent trends in management. J Conserv Dent. 2010;13:218–224.
- Holland GR, Narhi MN, Addy M, Gangarosa L, Orchardson R. Guidelines for the design and conduct of clinical trials on dentine hypersensitivity. J Clin Periodontol. 1997;24:808–813.
- Orchardson R, Collins WJ. Thresholds of hypersensitive teeth to 2 forms of controlled stimulation. J Clin Periodontol. 1987;14:68–73.
- Strassler H, Serio F. Dentinal hypersensitivity: etiology, diagnosis and management. Available at: www.ineedce.com/ courses/ 1728/ PDF/ DentinalHypersensitivity.pdf. Accessed March 25, 2013.
- Rees JS. The prevalence of dentine hypersensitivity in general dental practice in the UK. J Clin Periodontol. 2000;27:860–865.
- Chabanski MB, Gillam DG, Bulman JS, Newman HN. Prevalence of cervical dentine sensitivity in a population of patients referred to a specialist periodontology department. J Clin Periodontol. 1996;23:989–992.
- von Troil B, Needleman I, Sanz M. A systematic review of the prevalence of root sensitivity following periodontal therapy. J Clin Periodontal. 2002;52(Suppl):375–376.
- Orchardson R, Cadden SW. An update on the physiology of the dentine pulp complex. Dent Update. 2001;28:208–209.
- Winston AE, Charig AJ, Thong S. Mechanism of action of a desensitizing fluoride toothpaste delivering calcium and phosphate ingredients in the treatment of dental hypersensitivity. Part III: Prevention of dye penetration through dentin vs a calcium- and phosphate-free control. Compend Contin Educ Dent. 2010;31:46–52.
- Chidchuangchai W, Vongsavan N, Matthews B. Sensory transduction mechanisms responsible for pain caused by cold stimulation of dentine in man. Arch Oral Biol. 2007;52:154–160.
- Miglani S, Aggarwal V, Ahuja B. Dentin hypersensitivity: Recent trends in management. J Conserv Dent. 2010;13:218–224.2007;52:154–160.
- Addy M. Dentine hypersensitivity: new perspectives on an old problem. Int Dent J. 2002:52:367–376.
- Pashley DH, Tay FR, Haywood VB, Collins MC, Drisko CL. Dentin hypersensitivity: Consensus-based recommendations for the diagnosis and management of dentin hypersensitivity. Inside Dentistry. 2008;4(Suppl):I–35.
- Jacobsen PL, Bruce G. Clinical dental hypersensitivity: understanding the causes and prescribing a treatment. J Contemp Dent Pract. 2001;2:1–12.
- Aparna S, Setty S, Thakur S. Comparative efficacy of two treatment modalities for dentinal hypersensitivity: A clinical trial. Indian J Dent Res. 2010;21:544–548.
- Willis S. A patient-centered approach. Dimensions of Dental Hygiene. 2011;9(7):32–36.
- Hurlbutt M. Caries management with calcium phosphate. Dimensions of Dental Hygiene. 2010;8(10):40–46.
- Bittencourt S, Del Peloso Ribeiro E, Sallum EA, Sallum AW, Nociti FH Jr, Casati MZ. Comparative 6-month clinical study of a semilunar coronally positioned flap and subepithelial connective tissue graft for the treatment of gingival recession. J Periodontol. 2006;77:174–181.
From Dimensions of Dental Hygiene. April 2013; 11(4): 38–39, 41–42.

