
The Role of Interleukin-6 in Oral Diseases
The future of oral health care may include the diagnosis and modulation of this cytokine as a strategy for treatment and to improve patient outcomes.
Interleukin-6 (IL-6) is a signaling protein (cytokine) produced by several cells, including macrophages, neutrophils, keratinocytes, and fibroblasts, in response to stimuli, such as infection and trauma (Figure 1).1–3 IL-6 cell signals are transmitted to target cells through a surface receptor. The soluble IL-6 receptor then widens the group of cells responsive to IL-6.4 IL-6 stimulates a number of biological processes, including antibody (and auto-antibody) production, activation of T cells, B cell differentiation, increase in acute phase proteins, hematopoiesis, induction of angiogenesis, vascular permeability, and differentiation of osteoclasts.5,6 Therefore, IL-6 has important effects in the response to microbial insults, acting not only as an anti-inflammatory agent (eg, in the down-regulation of neutrophil recruitment and proinflammatory cytokine expression),7,8 but also as a proinflammatory agent (eg, in the induction of acute-phase reactants by the liver) when the inflammatory process becomes chronic.4 Variability in individuals’ ability to synthesize and release IL-6 may affect the susceptibility, development, and progression of a number of autoimmune and inflammatory diseases, such as atherosclerosis and rheumatoid arthritis (Table 1), and malignancies, including myeloma and mesothelioma.9
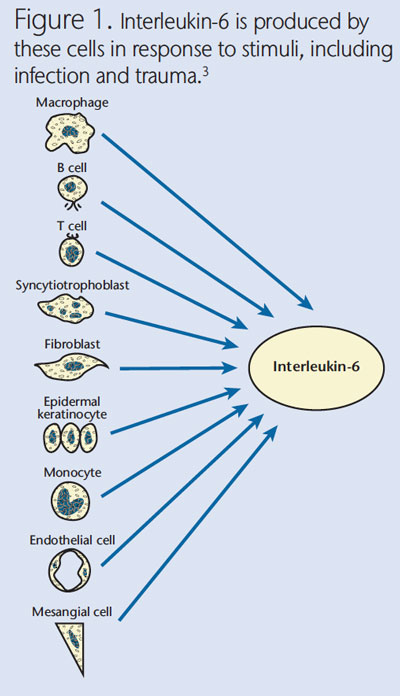
ROLE OF INTERLEUKIN-6 IN ORAL INFLAMMATORY DISEASES
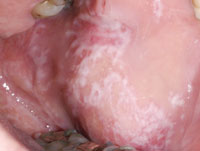
Many oral diseases, including gingivitis, periodontitis, pulpitis, and oral lichen planus (Figure 2) are inflammatory in nature. In periodontal diseases, the presence of microbes triggers an inflammatory-immune response mediated through alterations in the vascular network and exudation of gingival crevicular fluid (GCF), carrying inflammatory cells and plasma cells that eventually lead to tissue destruction.10 This inflammatory response is mediated by cytokines, such as IL-1, IL-6, and tumor necrosis factor alpha (TNF-?).11,12 In pulpitis, peptidoglycans from Gram-positive bacteria, such as Lactobacillus casei, enhance IL-6 production from human pulp cells in a time- and dose- dependent manner.13 In oral lichen planus, the subepithelial inflammatory cells infiltrate, consisting primarily of T cells and macrophages,14 which can lead to increases in the expression of other proinflammatory cytokines, including IL-6.15
PRESENCE OF INTERLEUKIN-6 IN INFLAMED ORAL TISSUES
Histological analyses revealed the presence of IL-6 in endothelial cells, macrophages, and periodontal ligament fibroblasts in response to periodontopathogenic bacteria,16–18 as well as in dental granulomas.19 Furthermore, IL-6 levels have been detected in GCF and saliva at higher levels in patients with gingivitis and periodontitis compared to healthy subjects.20–22 High levels of IL-6 have also been identified in inflamed pulp and periapical lesions compared to healthy pulp, especially in symptomatic lesions.23,24 Increased concentrations of IL-6 in saliva have been found in patients with oral lichen planus—especially severe forms—compared to healthy controls.15
IL-6 may also act as a signal transducer, activator of transcription, and as a growth factor for human keratinocytes and cancer cells.10,25,26 An in vitro study showed that IL-6 can stimulate oral squamous carcinoma cells to increase secretion of matrix metalloproteinases 1 and 9, which play a major role in infiltrative growth, metastasis, and the treatment of cancer.27 A recent study showed that IL-6 can also promote tumor growth by causing DNA methylation changes, which can lead to changes in the gene expression of oral cancer cells.28 As further proof of its possible effects on oral cancer, IL-6 levels in serum and saliva of patients with oral cancer were higher than those of control subjects.29,30
Altered IL-6 expression has also been detected in cases of oral mucosal ulceration caused by hereditary autoinflammatory syndromes that include periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis, familial mediterranean fever, as well as in recurrent aphthous stomatitis and Sjögren’s syndrome (see sidebar).31,32
INDIVIDUAL SUSCEPTIBILITY TO HIGHER INTERLEUKIN-6 PRODUCTION
The magnitude of inflammatory reactions seems to be related to common genetic variants called single nucleotide polymorphisms. In other words, individuals react with varying levels of inflammatory responses when faced with the same bacterial stimulation. Genetic variants in the IL-6 gene have been associated with IL-6 production and the subsequent response to infection.33,34 Therefore, it comes as no surprise that genetic variants affecting the ability to synthesize IL-6 may also predispose individuals to pathogenic oral bacteria colonization35 and oral diseases, such as gingivitis,36 chronic and aggressive periodontitis,35,37,38 symptomatic periapical lesions,39 and oral lichen planus.40 However, these results are related to specific populations and cannot be generalized until they are validated by large independent studies.
IS THERE A THERAPEUTIC PATHWAY TO TARGET INTERLEUKIN-6 IN ORAL DISEASES?
Targeted pharmacologic therapy aimed at reducing IL-6 is currently being used in the treatment of inflammatory diseases, such as rheumatoid arthritis and Crohn’s disease. These treatments use humanized monoclonal antibodies to neutralize IL-6 activities.11,41 In the future, there may be a therapy that neutralizes IL-6 signals—improving the treatment of oral diseases that would benefit from a reduction in IL-6 levels.42,43 However, significant side effects, including infection and increased cholesterol levels, limit their use at this stage.10

CONCLUSIONS
IL-6, together with other cytokines and active-phase reactants, modulates the response to oral bacteria. An excessive IL-6 response may contribute to the development of a chronic inflammatory lesion, resulting in loss of periodontal ligament and alveolar bone. This might happen through IL-6’s tissue-degradation effects on connective tissue and bone—mediated by metalloproteinases and osteoclasts, activation of T cells, or amplification of the inflammatory cascade.44 Local IL-6 production is probably at the source of the inflammatory response, mainly through macrophages, neutrophils, endothelial cells, and fibroblasts.22,23 Indeed, prolonged excessive release of IL-6 (which can be triggered by oral bacteria such as Aggregatibacteractinomycetemcomitans and Lactobacillus casei, and sustained by a hyperinflammatory genotype) can translate into persistent inflammation and tissue damage through proteases, osteoclasts, and methylation changes.13,16,28 The role that IL-6 plays as a growth factor in oral cancers is becoming increasingly clear. Potential diagnostic chairside tests and IL-6-modulating strategies could become available in the future that may significantly improve the efficacy of oral disease management.
ACKNOWLEDGEMENTS
PHOTOGRAPHY: ALFRED PASIEKA/PHOTO RESEARCHERS INC
FIGURE 2: DR. P. MARAZZI/PHOTO RESEARCHERS INC
Xerostomia and Autoinflammatory Diseases
 Interleukin-6 (IL-6) is thought to play a role in many autoinflammatory syndromes, including periodic fever, aphthous stomatitis, pharyngitis, cervical adenitis, familial mediterranean fever, and Sjögren’s syndrome. Besides a connection to IL-6, these diseases have something else in common—they can all cause xerostomia. While sometimes viewed as an annoying side effect of serious autoinflammatory disorders, xerostomia can exert significant deleterious effects on oral health. It can interfere with people’s ability to talk and swallow. Difficulty swallowing can lead to malnutrition as people compensate by limiting the types of foods they consume. The risk of infection—including bacterial, fungal, and viral—is elevated. If untreated, dry mouth can lead to the destruction of the oral cavity caused by rampant caries and periodontal infection. Dental hygienists are at the forefront of xerostomia detection and treatment. They need to provide their patients with strategies for addressing dry mouth, as well as emphasizing the importance of treatment for this oral health problem. Xerostomia can be effectively managed by implementing the following therapies: salivary stimulants and
Interleukin-6 (IL-6) is thought to play a role in many autoinflammatory syndromes, including periodic fever, aphthous stomatitis, pharyngitis, cervical adenitis, familial mediterranean fever, and Sjögren’s syndrome. Besides a connection to IL-6, these diseases have something else in common—they can all cause xerostomia. While sometimes viewed as an annoying side effect of serious autoinflammatory disorders, xerostomia can exert significant deleterious effects on oral health. It can interfere with people’s ability to talk and swallow. Difficulty swallowing can lead to malnutrition as people compensate by limiting the types of foods they consume. The risk of infection—including bacterial, fungal, and viral—is elevated. If untreated, dry mouth can lead to the destruction of the oral cavity caused by rampant caries and periodontal infection. Dental hygienists are at the forefront of xerostomia detection and treatment. They need to provide their patients with strategies for addressing dry mouth, as well as emphasizing the importance of treatment for this oral health problem. Xerostomia can be effectively managed by implementing the following therapies: salivary stimulants and
substitutes, oral moisturizers, professional and at-home fluoride use, chemotherapeutic mouthrinses and toothpastes, and products designed to treat desensitization and pain control.
REFERENCES
- Kishimoto T, Akira S, Narazaki M, Taga T. Interleukin-6 family of cytokines and Gp130. Blood. 1995;86:1243–1254.
- Matsuki Y, Yamamoto T, Hara K. Detection of inflammatory cytokinemessenger RNA (mRNA)-expressing cells in human inflamed gingiva by combined in situ hybridization and immuno histochemistry. Immunology.1992;76:42–47.
- Cerruto L. Non-TNF targeting biologics for rheumatoid arthritis.Rheumatology. Livingston, NJ: Projects in Knowledge; 2012.
- Jones KG, Brull DJ, Brown LC, et al. Interleukin-6 (IL-6) and the prognosis of abdominal aortic aneurysms. Circulation. 2001;103:2260–2265.
- Hirano T, Matsuda T, Turner M, et al. Excessive production of interleukin-6/b cell stimulatory factor in rheumatoid-arthritis. Europ J Immunol.1998;18:1797–1801.
- Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH.Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979.
- Schindler R, Mancilla J, Endres S, Ghorbani R, Clark SC, Dinarello CA.Correlations and interactions in the production of interleukin-6 (IL-6), IL-1,and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6suppresses IL-1 and TNF. Blood. 1990;75:40–47.
- Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF et al. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. 1998;101:311–320.
- Nishimoto N. Interleukin-6 as a therapeutic target in candidate inflammatory diseases. Clin Pharmacol Ther. 2010;87:483–487.
- Page RC, Schroeder HE. Pathogenesis of inflammatory periodontal disease. A summary of current work. Lab Invest. 1976;34:235–249.
- Howells GL. Cytokine networks in destructive periodontal disease. Oral Dis.1995;1:266–270.
- Graves DT, Cochran D. The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J Periodontol.2003;74:391–401.
- Matsushima K, Ohbayashi E, Takeuchi H, Hosoya S, Abiko Y, Yamazaki M.Stimulation of interleukin-6 production in human dental pulp cells by peptidoglycans from Lactobacillus casei. J Endod. 1998;24:252–255.
- Lodi G, Scully C, Carrozzo M, Griffiths M, Sugerman PB, Thongprasom K.Current controversies in oral lichen planus: Report of an internationalconsensus meeting. Part 2. Clinical management and malignanttransformation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.2005;100:164–178.
- Gu GM, Martin MD, Darveau RP, Truelove E, Epstein J. Oral and serum IL-6levels in oral lichen planus patients. Gu GM, Martin MD, Darveau RP, Truelove E, Epstein J. Oral and serum IL-6levels in oral lichen planus patients. Oral Surg Oral Med Oral Pathol OralRadiol Endod. 2004;98:673–678. 2004;98:673–678.
- Belibasakis GN, Johansson A, Wang Y, et al, Cytokine responses of human gingival fibroblasts to Actinobacillus actinomycetemcomitans cytolethaldistending toxin. Cytokine. 2005;30:56–63.
- Patil C, Rossa C, Kirkwood KL. Actinobacillus actinomycetemcomitanslipopolysaccharide induces interleukin-6 expression through multiple mitogen-activated protein kinase pathways in periodontal ligamentfibroblasts. Oral Microbiol Immunol. 2006;21:392-398.
- Rogers JE, Li F, Coatney DD, et al. Actinobacillus actinomycetemcomitanslipopolysaccharide-mediated experimental bone loss model for aggressiv eperiodontitis. J Periodontol. 2007;78:550–558.
- De Sa AR, Pimenta FJGS, Dutra WO, Gomez RS. Immunolocalization ofinterleukin 4, interleukin 6, and lymphotoxin alpha in dental granulomas.Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:356–360.
- Johanssen A, Bjurshammar N, Gustafsson A. The influence of academic stress on gingival inflammation. Int J Dent Hyg. 2010;8:22–27.
- Becerik S, Ozçaka O, Nalbantsoy A, et al, Effects of menstrual cycle on periodontal health and gingival crevicular fluid markers. J Periodontol. 2010;81:673–681.
- Shimada Y, Komatsu Y, Ikezawa-Suzuki I, Tai H, Sugita N, Yoshie H. The effect of periodontal treatment on serum leptin, interleukin-6, and C-reactive protein. J Periodontol. 2010;81:1118–1123.
- Barkhordar RA, Hayashi C, Hussain MZ. Detection of interleukin-6 inhuman dental pulp and periapical lesions. Endod Dent Traumatol.1999;15:26–27.
- Prso IB, Kocjan W, Simic H, et al. Tumor necrosis factor-alpha and interleukin 6 in human periapical lesions. Mediators Inflamm. 2007:38210.
- Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth,differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19:2548–2556.
- Barton BE. Interleukin-6 and new strategies for the treatment of cancer,hyper proliferative diseases and paraneoplastic syndromes. Expert Opin Therargets. 2005;9:737–752.
- Sundelin K, Roberg K, Grenman R, Hakansson L. Effects of cytokines on matrix metalloproteinase expression in oral squamous cell carcinoma in vitro.Acta Otolaryngol. 2005;125:765–773.
- Gasche JA, Hoffmann J, Boland CR, Goel A. Interleukin-6 promotest umorigenesis by altering DNA methylation in oral cancer cells. Int J Cancer.2011;129:1053–1063.
- Chen Z, Malhotra PS, Thomas GR, et al. Expression of proinflammatory and proangiogenic cytokines in patients with head and neck cancer. Clin Cancer Res. 1999;5:1369–1379.
- Bigbee WL, Grandis JR, Siegfried JM. Multiple cytokine and growth factorserum biomarkers predict therapeutic response and survival in advanced stage head and neck cancer patients. Clin Cancer Res. 2007;13:3107–3108.
- Lachmann HJ, Quartier P, So A, Hawkins PN. The emerging role of interleukin-1? in auto inflammatory diseases. Arthritis Rheum.2011;63:314–324.
- Roescher N, Tak PP, Illei GG. Cytokines in Sjögren’s syndrome. Oral Dis.2009;15:519–526.
- Fishman D, Faulds G, Jeffery R, et al. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest.1998;102:1369–1376.
- Poikonen K, Lajunen T, Silvennoinen-Kassinen S, Leinonen M, Saikku P.Effects of CD14, TLR2, TLR4, LPB, and IL-6 gene polymorphisms on Chlamydia pneumoniae growth in human macrophages in vitro. Scand J Immunol.2009;70:34–39.
- Nibali L, Donos N, Henderson B. Periodontal infectogenomics. J Med Microbiol. 2009;58:1269–1274.
- Holla LI, Musilova K, Vokurka J, et al. Association of interleukin-6 (IL-6) haplotypes with plaque-induced gingivitis in children. Acta Odont Scandin.2008;66:105–112.
- Trevilatto PC, Scarel-Caminaga RM, de Brito RB, de Souza AP, Line SRP.Polymorphism at position-174 of IL-6 gene is associated with susceptibility toc hronic periodontitis in a Caucasian Brazilian population. J Clin Periodontol.2003;30:438–442.
- Franch-Chillida F, Nibali L, Madden I, Donos N, Brett P. Association between interleukin-6 polymorphisms and periodontitis in Indian nonsmokers.J Clin Periodontol. 2010;37:137–144.
- De Sa AR, Moreira PR, Xavier GM, et al. Association of CD14, IL1B, IL6, IL10and TNFA functional gene polymorphisms with symptomatic dentalabscesses. Int Endod J. 2007;40:563–572.
- Xavier GM, de Sa AR, Guimaraes AL, da Silva TA, Gomez RS. Investigation of functional gene polymorphisms interleukin-1beta, interleukin-6,interleukin-10 and tumor necrosis factor in individuals with oral lichen planus.J Oral Pathol Med. 2007;36:476–481.
- Smolen JS, Aletaha D. Interleukin-6 receptor inhibition with tocilizumab and attainment of remission: the role of acute phase reactants. ArthritisRheum. 2011;63:43–52.
- Hong DS, Angelo LS, Kurzrock R. Interleukin-6 and its receptor in cancer:implications for translational therapeutics. Cancer. 2007;110:1911–1928.
- Shinriki S, Jono H, Ota K, Uesda M, Kudo M, Ota T, et al. Humanized anti interleukin-6 receptor antibody suppresses tumor angiogenesis and in vivo growth of human oral squamous cell carcinoma. Clin Cancer Res.2009;15:5426–5434.
- Nibali L, Fedele S, D’Aiuto F, Donos N. Interleukin-6 in oral diseases: areview. Oral Dis. 2012;18:236–243.
From Dimensions of Dental Hygiene. January 2013; 11(1): 28, 30, 32–34.

