 OCSKAYMARK / ISTOCK / THINKSTOCK
OCSKAYMARK / ISTOCK / THINKSTOCK
The New Generation of Blood Thinners
Special considerations are needed when providing dental care to patients taking novel oral anticoagulants.
By Sandra Stramoski, RDH, MSDH, and Anna Matthews, RDH, MS
This course was published in the October 2016 issue and expires October 31, 2019. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
The advantages of NOACs, also called direct oral anticoagulant drugs, include targeted mechanisms of action, predictability, few drug interactions, and the need for less frequent dosing and monitoring.2 Indications for their use include prevention of stroke and systemic blood clots in patients with nonvalvular atrial fibrillation, deep vein thrombosis, and pulmonary embolism, particularly following hip and knee replacement surgery.
With more than 12 million prescriptions of the NOAC dabigatran filled in its first 5 years, and 5 million prescriptions for rivaroxaban in a 12-month period from 2013 to 2014, the need for clarity on patient management and dental practice implications is paramount.
BLOOD COAGULATION PROCESS
Blood fluidity within the vasculature is maintained by complex physiological systems that preserve the balance between blood coagulation and fibrinolysis. The coagulation system activates in response to vascular injury and prevents hemorrhage, while the fibrinolytic system dissolves blood clots and intravascular thrombi, preventing thrombosis. In health, these systems are in balance, supported by factors within the endothelial wall, blood platelets, and plasma coagulation factors.
To prevent the formation of intravascular thrombi, the undamaged epithelium releases chemical mediators, such as prostacyclin and nitric oxide, that inhibit platelet aggregation. Nitric oxide is also a potent vasodilator and an important signaling molecule in the cardiovascular system. Its ability to relax smooth muscle and dilate arteries and veins is used in a number of cardiac and other medications.3 When the endothelium is damaged, the von Willebrand factor (vWF) in the vascular epithelium interacts with the glycoprotein 1b receptors on the platelets’ surface, resulting in their activation and adhesion to the exposed collagen fibers within the vascular wall. Activated platelets release a variety of mediators that enhance activation, adhesiveness, and attraction of more platelets, as well as vasoconstriction. Importantly, activated platelets change their shape and increase their affinity for fibrinogen by conformational change in two glycoprotein receptors: GPIIa and GPIIIb. Fibrinogen binding to these receptors results in cross-linking of the multiple platelets and further aggregation.
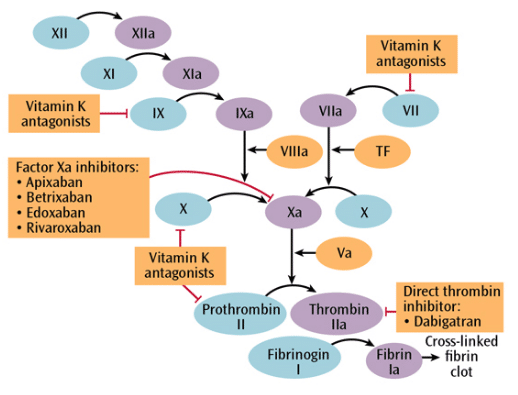
Tissue factors released during the endothelial injury and mediators released by the activated platelets stimulate the coagulation cascade (Figure 1), resulting in the formation of thrombin, which then catalyzes the hydrolysis of fibrinogen to fibrin—an important component binding the platelets to form a clot. While this process is beneficial to tissue injury by quickly achieving hemostasis, alteration of the balance between blood coagulation and fibrinolysis in favor of coagulation may be an inappropriate response. This is because of changes within the cardiovascular system caused by disease, atherosclerosis, and lesions in the vascular walls, or following stent placement. Thrombi that form in the arteries and veins can lead to tissue ischemia and vascular occlusion, resulting in tissue death. They can also dislodge from the vascular wall and result in embolism, leading to serious complications such as stroke, heart attack, deep vein thrombosis, and pulmonary embolism.
Drugs used in antithrombotic therapy that target key steps in clot formation include: antiplatelet drugs, which inhibit platelet activation or aggregation; anticoagulants, which decrease fibrin formation by blocking various steps in the coagulation cascade; and fibrinolytic agents, which degrade fibrin and dissolve thrombi. While antiplatelet drugs and anticoagulants that target each step of clot formation are used to prevent thrombogenesis, they have no effect on the existing clots, except for limiting their progression. Only fibrinolytic agents can dissolve existing thrombi.4 Indications for antithrombotic therapy using antiplatelet agents and anticoagulants, include the prevention of thromboembolitic events and stroke in atrial fibrillation and other cardiac arrhythmias; venous thromboembolism; acute coronary syndrome and myocardial infarction; pulmonary hypertension; and cardiac valve disease and prosthetic valve placement.5 Additionally, long-term dual antiplatelet therapy is required for the prevention of stent thrombosis following the implantation of coronary stents.6 In some patients, triple therapy using two antiplatelet medications (aspirin and clopidogrel or prasugrel) and an anticoagulant is necessary to prevent stroke and other coronary events after stent implantation.7 While antithrombotic agents have different mechanisms of action, they increase the risk of bleeding correlated to the potency of the drug.
WARFARIN
Warfarin is a vitamin K antagonist that is used as an oral anticoagulant. It is the oldest, most extensively studied oral anticoagulant and, arguably, the most problematic. It requires initial dose adjustment and frequent monitoring due to patients’ sensitivity based on their genetic variations in the hepatic enzymes metabolizing the drug, comorbidity with renal or hepatic disease, and age (sensitivity to warfarin increases with age).1 Due to numerous drug interactions resulting in either potentiation of the warfarin effect—hence increased risk of bleeding—or its decreased effect, concomitant use with many medications commonly prescribed in dentistry should be avoided. These include azole antifungals, metronidazole, sulfamethoxazole, nonsteroidal anti-inflammatory drugs, and aspirin. Acetaminophen, macrolide antibiotics, tetracyclines, levofloxacine, and cephalosporins should be used with caution.8
Warfarin’s onset of action is 24 hours to 72 hours, and it can take up to 7 days to achieve full therapeutic effect. Half-life elimination is highly variable, ranging from 20 hours to 40 hours and duration of action ranges from 2 days to 5 days. This means that advance discontinuation and bridge therapy with short-acting heparin or its analogs are required in cases of anticipated excessive bleeding during surgery.4,5 The one notable advantage of warfarin is its ability for reversal of excessive anticoagulation and resultant bleeding by vitamin K.1
The International Normalized Ratio (INR) is used to monitor anticoagulant therapy by determining the relationship of the patient’s prothrombin time (PT) to a PT of a normal plasma sample. NOACs do not require INR monitoring. The therapeutic range of INR for most procedures is 2 to 3; higher numbers indicate increased anticoagulation and bleeding risk.1 Despite the concern about increased bleeding during periodontal and dental procedures in patients on anticoagulant therapy,13,14 a number of clinical studies,9–11 systematic reviews12 and meta-analyses13 found that continuing warfarin without any dose adjustment before dental procedures did not increase the risk of clinically important bleeding. However, discontinuing anticoagulant therapy placed the patients at risk for thromboembolic events and death.9 Nevertheless, there is concern about increased bleeding in patients on anticoagulant therapy.13,14
NOVEL ORAL ANTICOAGULANTS
Four NOACs are currently approved by the United States Food and Drug Administration (FDA): dabigatran, rivaroxaban, apixaban, and edoxaban. Dabigatran is a direct thrombin inhibitor. Unlike warfarin, dabigatran has a stronger affinity for thrombin and a lower affinity for and possibly inhibitory action on other enzymes in this process (Figure 1). Specifically, dabigatran prevents the transformation of fibrinogen to fibrin, therefore inhibiting clot formation as well as platelet activation.2,15 Dabigatran is FDA approved to treat nonvalvular atrial fibrillation and prevent deep vein thrombosis and pulmonary embolism. It is contraindicated in patients with prosthetic heart valves and pathological bleeding. Common adverse reactions include gastritis-like symptoms and bleeding.16
In the premarket drug trial on dabigatran vs warfarin among 18,000 patients with atrial fibrillation, dabigatran was either equally or more effective in reducing stroke and systemic embolism than warfarin, but produced slightly higher values of patients with myocardial infarction.17 Subsequent studies measuring these outcomes, as well as reported adverse effects, have not prompted changes in recommendations for use.18 In recent reports, the risk of myocardial infarction has proven to be more similar in the two drugs, with an increased advantage of dabigatran over warfarin in stroke and embolism prevention—albeit a slightly higher risk of gastrointestinal bleeding.18 The risk of bleeding events has been shown to increase with age.19
Dabigatran lacks the narrow therapeutic index and lower safety profile of warfarin. These factors have made it among the top 100 prescribed drugs since 2012. Due to its much shorter half-life (8 hours to 15 hours) than warfarin (20 hours to 40 hours), bleeding events, if not critical, were previously reversed by medically supervised cessation of the drug, hemodialysis, or local measures such as activated charcoal. Introduction of the dabigatran-specific reversal agent idarucizumab has revolutionized this process, making emergency surgical procedures and reversal of serious bleeding events more predictably achieved.20
Rivaroxaban, apixaban, and edoxaban are blood clotting factor Xa inhibitors, exerting their effects earlier than dabigatran in the blood coagulation cascade (Figure 1). These agents inhibit the activation of prothrombin to thrombin—the factor upon which dabigatran exerts its inhibitory effects, thereby inhibiting clot formation and platelet aggregation. All three drugs are FDA approved for nonvalvular atrial fibrillation, deep vein thrombosis, pulmonary embolism, and deep vein thrombosis and pulmonary embolism prophylaxis following knee and hip replacement surgery.21–25
A 2012 meta-analysis of three large-scale phase III randomized control trials involving more than 40,000 patients compared dabigatran, rivaroxaban, and apixaban with warfarin for efficacy and safety. The meta-analysis found a decreased risk with the NOACs for all-cause stroke, hemorrhagic stroke, all-cause mortality, vascular mortality, and lower risk for intracranial bleeding than warfarin.26 A 2015 meta-analysis that included all four currently available NOACs compared the drugs for the treatment and prevention of venous thromboembolism and found they were equally effective. Apixaban and dabigatran had the most favorable reduction of risk of clinically relevant nonmajor bleeding events when compared to rivaroxaban.27 Finally, a 2016 analysis compared apixaban to the other three NOACs and warfarin, concluding that apixaban had the best safety and efficacy profile.24
ADVERSE EFFECTS
The most common adverse effects of NOACs are extensions of their therapeutic effects, namely bleeding events. Patients on additional anticoagulant or antiplatelet therapy, such as aspirin; those with medical comorbidities; and those older than 75 are at increased risk for bleeding events.28
Drug interactions with the direct oral anticoagulants are far fewer than warfarin. Clinicians who anticipate prescribing antibiotics, antivirals, antifungals, or analgesics, or recommending over-the-counter pain relievers to individuals taking any antithrombotic drugs, should consult the agent-specific medication guide or drug information sources for detailed precautions.28 Selective serotonin reuptake inhibitors were recently added to the list of interactions for rivaroxaban.21
BLOOD MONITORING
A disadvantage of NOACs is the limited availability of antidotes to be used in the event of emergency surgery or uncontrolled, life-threatening bleeding. Warfarin is effectively reversed by the administration of vitamin K; heparin and LMWH by protamine; and various agents for other anticoagulants have been used successfully for many years.19,29 Dabigatran is the first and only NOAC to have an FDA-approved antidote to date: idarucizumab. It has been shown to safely and completely reverse its anticoagulant effects.20 At this point, Andexanet-alpha has undergone in vitro trials and is in phase three of in vivo trials as a universal factor Xa antidote.30
Routine blood monitoring is not necessary with NOACs because of the more predictable effects of a fixed dose. Various blood tests for coagulation are available and may be used in an event at uncontrolled bleeding, suspected thrombosis, or overdose.31 Currently, the thrombin time and activated partial thromboplastin time tests are considered accurate enough in reassuring safe emergency surgical procedures.32
TREATMENT CONSIDERATIONS
Discontinuing antithrombotic medications before invasive dental procedures is indicated when prolonged bleeding is anticipated and only under the prescribing physician’s orders.21–23,33 Current guidelines support continuation of warfarin. Stopping the use of NOACs is not indicated for dental hygiene procedures, including periodontal debridement and scaling and root planing.34 Medical consultation is advised when patients require oral/periodontal surgery. Studies that included NOACs and warfarin have shown that timing a procedure further out from NOAC dosing, the use of atraumatic techniques, and employing local hemostatic measures resulted in minimal adverse outcomes.4,10,11,34
Patient considerations include assessing stabilization of comorbidities, such as diabetes and renal dysfunction, which may prolong drug clearance, as post-treatment bleeding events and slower wound healing are more likely. Conservative measures to eliminate oral inflammation and control disease are prudent in all patients with increased bleeding risk.
CONCLUSION
The widespread use of NOACs has changed the landscape of patient treatment in the dental office. Continued development of more predictable, targeted agents that do not require routine blood monitoring will allow millions of people to reduce life-threatening risks more successfully. Older agents, such as warfarin, will continue to be used until new pharmacological interventions replace them. New drugs and devices that enable point-of-care assessment of their effects on coagulation levels are under development to ensure the safe delivery of surgical procedures and traditional treatment in the dental setting.
REFERENCES
- Brunton LL, Chabner BA, Knollmann, Björn C. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. New York: McGraw Hill; 2011.
- Elad S, Marshall J, Meyerowitz C, Connolly G. Novel anticoagulants: general overview and practical considerations for dental practitioners. Oral Dis. 2016;22:23–32.
- Wynn RL, Meiller TF, Crossley HF. Drug Information Handbook for Dentistry. 20th ed. Hudson, Ohio: Lexi-Comp; 2014.
- Becker DE. Antithrombotic drugs: pharmacology and implications for dental practice. Anesth Prog. 2013;60:72–80.
- Fakhri H, Janket S, Jackson E, Baird A, Dinnocenzo R, Meurman J. Tutorial in oral antithrombotic therapy: Biology and dental implications. Med Oral Patol Oral Cirugia Bucal. 2013:18:e461–472.
- Kovacic JC, Lee P, Karajgikar R, et al. Safety of temporary and permanent suspension of antiplatelet therapy After drug eluting stent implantation in contemporary “real-world” practice: Antiplatelet cessation after DES. J Intervent Cardiol. 2012;25:482–492.
- Lamberts M, Olesen JB, Ruwald MH, et al. Bleeding after initiation of multiple antithrombotic drugs, including triple therapy, in atrial fibrillation patients following myocardial infarction and coronary intervention: A nationwide cohort study. Circulation. 2012;126:1185–1193.
- Firriolo FJ, Hupp WS. Beyond warfarin: the new generation of oral anticoagulants and their implications for the management of dental patients. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113:431–441.
- Wahl MJ. Dental surgery in anticoagulated patients. Arch Intern Med. 1998;158:1610.
- Bakathir AA. Minor oral surgery procedures in patients taking warfarin: A 5-year retrospective study at Sultan Qaboos University Hospital, Sultanate of Oman. Sultan Qaboos Univ Med J. 2009;9:279–286.
- Pereira CM, Gasparetto PF, Carneiro DS, Corrêa MEP, Souza CA. Tooth Extraction in patients on oral anticoagulants: Prospective study conducted in 108 Brazilian patients. ISRN Dent. 2011;2011:1–4.
- Dunn AS, Turpie AGG. Perioperative management of patients receiving oral anticoagulants: a systematic review. Arch Intern Med. 2003;163:901–908.
- Nematullah A, Alabousi A, Blanas N, Douketis JD, Sutherland SE. Dental surgery for patients on anticoagulant therapy with warfarin: A systematic review and meta-analysis. J Can Dent Assoc. 2009;75:41.
- Linnebur SA, Ellis SL, Astroth JD. Educational practices regarding anticoagulation and dental procedures in U.S. dental schools. J Dent Educ. 2007;71:296–303.
- Cheng JW, Barillari G. Non-vitamin K antagonist oral anticoagulants in cardiovascular disease management: evidence and unanswered questions. J Clin Pharm Ther. 2014;39:118–135.
- Pradaxa [package insert]. Ridgefiled, Connecticut. Boehringer Ingelheim Pharmaceuticals Inc; 2015.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151.
- United States Food and Drug Administration. Pradaxa (Dabigatran Etexilate Mesylate) Should Not Be Used in Patients With Mechanical Prosthetic Heart Valves. Available at: fda.gov/Drugs/DrugSafety/ ucm332912.htm. Accessed September 14, 2016.
- Suryanarayan D, Schulman S. Potential antidotes for reversal of old and new oral anticoagulants. Thromb Res. 2014;133:S158–S166.
- Abo-Salem E, Becker RC. Reversal of novel oral anticoagulants. Curr Opin Pharmacol. 2016;27:86–91.
- US Food and Drug Administration. Medication Guide Xarelto® Tablets. Available at: fda.gov/ downloads/Drugs/DrugSafety/UCM280333.pdf. Accessed September 14, 2016.
- US Food and Drug Administration. Medication Guide Eliquis® Tablets. Available at: fda.gov/downloads/Drugs/DrugSafety/UCM333961.pdf. Accessed September 14, 2016.
- US Food and Drug Administration. Medication Guide Savaysa Tablets. Available at: fda.gov/downloads/Drugs/DrugSafety/UCM467811.pdf. Accessed September 14, 2016.
- Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–817.
- Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992.
- Miller CS, Grandi SM, Shimony A, Filion KB, Eisenberg MJ. Meta-analysis of efficacy and safety of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus warfarin in patients with atrial fibrillation. Am J Cardiol. 2012;110:453–460.
- Cohen AT, Hamilton M, Mitchell SA, et al. Comparison of the novel oral anticoagulants apixaban, dabigatran, edoxaban, and rivaroxaban in the initial and long-term treatment and prevention of venous thromboembolism: Systematic review and network meta-analysis. PLoS One. 2015;10:e0144856.
- Fang LST. Pradaxa® and Xarelto®: Coming soon to your practice. Dental Economics. 2013;103(10):111–115.
- Tummala R, Kavtaradze A, Gupta A, Ghosh RK. Specific antidotes against direct oral anticoagulants: A comprehensive review of clinical trials data. Int J Cardiol. 2016;214:292–298.
- Lippi G, Sanchis-Gomar F, Favaloro EJ. Andexanet: Effectively reversing anticoagulation. Trends Pharmacol Sci. 2016;37:413–414.
- Tsantes AE, Kyriakou E, Ikonomidis I, et al. Comparative assessment of the anticoagulant activity of rivaroxaban and dabigatran in patients with nonvalvular atrial fibrillation: A noninterventional study. Medicine (Baltimore). 2016;95:e3037.
- Pinho-Gomes A-C, Hague A, Ghosh J. Management of novel oral anticoagulants in emergency and trauma surgery. Surgeon. 2016;14:234–239.
- US Food and Drug Administration. Medication Guide. Pradaxa Capsules. Available at: fda.gov/downloads/Drugs/DrugSafety/UCM231720.pdf. Accessed September 14, 2016.
- Costantinides F, Rizzo R, Pascazio L, Maglione M. Managing patients taking novel oral anticoagulants (NOAs) in dentistry: A discussion paper on clinical implications. BMC Oral Health. 2016;16:1–9.
From Dimensions of Dental Hygiene. October 2016;14(10):36–39.



