 DINA2001 / ISTOCK / THINKSTOCK (COMPOSITE BY JON FRAZE)
DINA2001 / ISTOCK / THINKSTOCK (COMPOSITE BY JON FRAZE)
Infection Control Update
Oral health professionals need to be familiar with The Centers for Disease Control and Prevention’s recently published summary of infection prevention practices in dental settings.
This course was published in the October 2016 issue and expires October 31, 2019. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Discuss the significance of the United States Centers for Disease Control and Prevention’s Summary of Infection Prevention Practices in Dental Settings.
- Explain the level of compliance with established guidelines by dental practices.
- List training and compliance resources.
The CDC Summary explains infection prevention principles and recommendations for dental settings. It also reaffirms standard precautions as the foundation for preventing transmission of infectious agents during patient care. The document includes a two-part checklist that can be used to evaluate compliance with CDC recommendations. The CDC Summary also provides links to the complete guidelines, other recommendations published since 2003, and source documents that readers can reference for more detailed information.
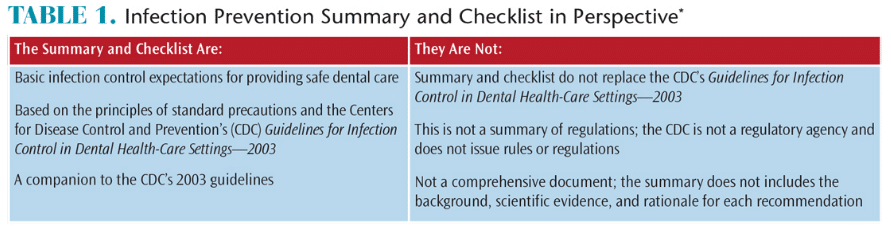
Working with the CDC, the Organization for Safety, Asepsis and Prevention (OSAP) has supported dissemination of the CDC Summary through its website (osap.org), presentations at professional meetings, and webinars. The website contains links to the CDC Summary and key resources, and also provides a clear explanation of what the CDC Summary is and is not (Table 1).8
BACKGROUND PERSPECTIVE
Since the release of the 2003 guidelines, the CDC’S Division of Oral Health has continued to review the scientific literature. In 2016, a review was conducted to identify documented reports of bloodborne pathogen transmission in dental settings since 2003. The reviewers examined summaries of all formal and informal reports from state and local health departments of suspected health care-associated human immunodeficiency virus (HIV) or hepatitis transmissions. It also reviewed the CDC’s Division of Hepatitis database.9
The authors identified three published reports of transmission of hepatitis B and C viruses in dental settings between 2003 and 2015. Two single-transmission events (from one patient to another) occurred in outpatient oral surgery practices.9 Another study documented the transmission of hepatitis B to three patients and two dental health care personnel (DHCP) in a large temporary dental clinic (Table 2).9
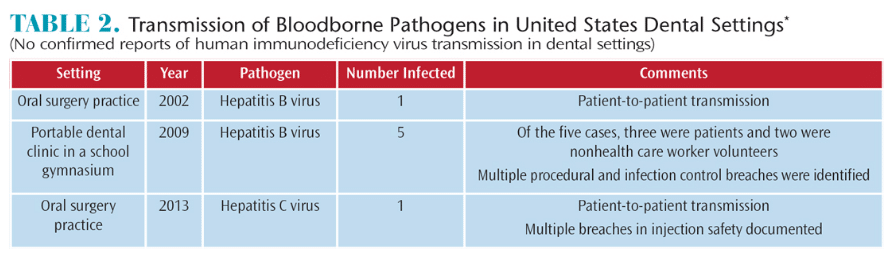
The authors concluded that reports of the transmission of bloodborne pathogens associated with dental health care settings is rare.9 The existence of these reports emphasizes the need to improve DHCPs’ understanding of basic principles of infection prevention and control, implementation of standard precautions.9
Research published in 2012 also supports the need for improved compliance with existing guidelines. In the report, CDC scientists studied the percentage of US dentists using four CDC infection control measures, and identified factors associated with the implementation of infection control recommendations.10 The American Dental Association collected the data by surveying a stratified random sampling of 6,825 US dentists.
The following recommendations are indicators for guideline adherence:
- Designating an infection control coordinator to monitor all infection control activities
- Using a separate water system for each dental unit that had been monitored at least once in the preceding 12 months to assess water quality
- Routinely documenting and referring individuals with percutaneous injuries for medical evaluation
- Using safer medical devices, such as safer syringes and scalpels, as mandated by the federal Occupational Safety and Health Administration
Based on a 49% response rate, the research showed that dentists in 34% of the practices surveyed had implemented none or one of the recommendations, 40% had implemented two, and 26% had implemented three or four. The two most frequently implemented recommendations were designating an infection control coordinator and always documenting percutaneous injuries.10 The likelihood of implementation was highest among dentists who:10
- Acknowledged the importance of infection control
- Had practiced dentistry for less than 30 years
- Correctly identified procedures that required the use of sterile water
- Had received more continuing education credits in infection control (as compared to less-compliant respondents)
- Worked in large practices
- Had at least three sources of instruction regarding the CDC guidelines
The authors concluded that implementation of the recommendations was neither complete nor uniform across practices. They further suggested strategies to raise awareness of the importance of infection prevention and increasing continuing education requirements as a means of improving implementation of the existing recommendations.10
The CDC’s Division of Oral Health published the CDC Summary to provide a resource to facilitate compliance.1
TRAINING AND COMPLIANCE RESOURCES
The CDC Summary is an excellent resource for DHCP education and training. It also serves as a reference for developing policies and standard operating procedures for infection prevention programs. Clinicians should consult the full guidelines for additional background information and the rationale and scientific evidence behind each recommendation.1
As noted, the CDC Summary includes a companion two-part checklist that can be used to periodically evaluate compliance with infection prevention practices. Along with the CDC Summary, the CDC also published a compendium document, Recommendations From the Guidelines for Infection Control in Dental Health-Care Settings—2003. A helpful resource for clinical use, the compendium is a reformatted, easy-to-use listing of the 2003 guidelines.11
The CDC Summary checklist—including a writable pdf checklist—and compendium are available online (Table 3). Additional infection prevention resources are listed in Table 4.
According to the CDC, the overarching goal of an infection prevention program is to provide a safe working environment that will reduce the risk of health care-associated infections among patients and DHCP, including volunteers.1,2 Program evaluation is an essential component of safe care, and the CDC guidelines describe evaluations as a “systematic way to ensure that infection prevention procedures are useful, feasible, ethical, and accurate.”2 In its Guidelines for Infection Control in Dental Health-Care Settings—2003, the CDC suggests elements regarding program evaluation (Table 5), and further states that a successful infection prevention program depends on:2
- Developing standard operating procedures
- Evaluating practices
- Routinely documenting adverse outcomes (eg, occupational exposures to blood) and work-related illnesses in DHCPs
- Monitoring health-care associated infections in patients
INFECTION PROTOCOL CHECKLISTS
The CDC Summary and two-part checklist are valuable tools for site-specific evaluation of compliance with CDC recommendations. These can be used as a component of an overall quality assurance or continuing quality improvement program. Section I is designed to facilitate evaluation of administrative policies and procedures, and the clinic’s or practice’s written infection prevention protocol. Section II can be used to observe and evaluate infection prevention and control practices by DHCP during the performance of their duties.<sup>1</sup> In addition to the CDC Summary checklist, other day-to-day operational checklists may be indicated. Checklists should be used for repeatable, recurring processes (eg, dental unit waterline treatment, biologic monitoring of the sterilizer, and routine sterilizer maintenance).
Checklists serve as reminders of the critical steps that must be completed during each procedure. It also provides a record that the proper procedure and steps were completed, and serves as documentation to investigate adverse events.
In addition to their utility in monitoring work practices and evaluating compliance with recommendations, checklists can be useful in identifying gaps in compliance, as well as in determining any necessary remediation. The latter may include modifying an existing written policy and protocol, developing new policies and procedures, changing a device or product, or targeted training for an individual or entire team.
Besides site-specific checklists and evaluation forms, dental practice settings may use additional checklists or audit forms from regulatory and accrediting agencies. Some state licensing boards, for example, use audit forms and checklists to assess compliance with CDC infection prevention recommendations. These forms and checklists may be available from state board websites. Other accrediting organizations—such as the Joint Commission on Healthcare Accreditation and state departments of public health—use checklists and audit forms to evaluate a site’s compliance with standards and regulations.
INFECTION PREVENTION COORDINATOR
To facilitate infection prevention program development, implementation, and evaluation, each practice setting should have a trained infection prevention coordinator. The recommendation is to assign at least one individual responsibility for coordinating the program. According to the CDC Summary, “The infection prevention coordinator should ensure that equipment and supplies (eg, hand hygiene products, devices to reduce percutaneous injuries, and personal protective equipment) are available, and should maintain communication with all staff members to address specific issues or concerns related to infection prevention.”1,2
Likewise, OSAP also recommends designating an infection prevention coordinator to manage and coordinate the infection control and safety program.12 In addition to providing a variety of resources, the organization lists suggested roles and responsibilities on its website.12 Depending on the practice setting, the infection prevention coordinator might be an individual, or, in larger settings, a committee with defined responsibilities. Alternately, a practice setting may choose to hire a consultant to help with program development or assessment, and serve as an ongoing resource to the on-site infection prevention coordinator.
In conclusion, a successful and compliant infection prevention program requires ongoing monitoring and evaluation. Designating an infection prevention coordinator to provide oversight, and using evaluation tools—such as the recently issued CDC Summary, checklist and compendium—are key to preventing infectious transmission in dental settings. Developing a true culture of safety requires a commitment to the program by all personnel, as demonstrated by day-to-day infection prevention practices.
REFERENCES
- United States Centers for Disease Control and Prevention. Summary of Infection Prevention Practices in Dental Settings: Basic Expectations for Safe Care. Available at: cdc.gov/oralhealth/ infectioncontrol/guidelines. Accessed September 1, 2016.
- US Centers for Disease Control and Prevention. Guidelines for infection control in dental health-care settings—2003. MMWR Recomm Rep. 2003;52(RR17):1–61.
- Siegel JD, Rhinehart E, Jackson M, Chiarello L, Healthcare Infection Control Practices Advisory Committee. US Centers for Disease Control and Prevention. 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings. Available at: cdc.gov/hicpac/pdf/isolation/isolation2007.pdf. Accessed September 1, 2016.
- US Centers for Disease Control and Prevention. CDC guidance for evaluating health-care personnel for hepatitis B virus protection and for administering post-exposure management. MMWR Recomm Rep. 2013;62(RR10):1–19.
- Summers CJ, Gooch BF, Marianos DW, Malvitz DM, Bond WW. Practical infection control in oral health surveys and screenings. J Am Dent Assoc. 1994;125:1213–1217.
- Bolyard EA, Tablan OC, Williams WW, et al. Guideline for infection control in healthcare personnel, 1998. Infect Control Hosp Epidemiol. 1998;19:407–463.
- U.S. Centers for Disease Control and Prevention. Immunization of health-care personnel: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60(RR07):1–45.
- Organization for Safety, Asepsis and Prevention. New CDC Summary and Checklist. Available at: osap.org/page/NewCDCSummary. Accessed September 1, 2016.
- Cleveland JL, Gray SK, Harte JA, Robison VA, Moorman AC, Gooch BF. Transmission of blood-borne pathogens in US dental health care settings: 2016 Update. J Am Dent Assoc. 2016;147:729–738.
- Cleveland JL, Bonito AJ, Corley TJ, et al. Advancing infection control in dental care settings: factors associated with dentists’ implementation of guidelines from the Centers for Disease Control and Prevention. J Am Dent Assoc. 2012;143:1127–1138.
- US Centers for Disease Control and Prevention. Recommendations From the Guidelines for Infection Control in Dental Health-Care Settings — 2003. Available at: cdc.gov/oralhealth/infectioncontrol/pdf/recommendations-excerpt.pdf. Accessed September 1, 2016.
- Organization for Safety, Asepsis and Prevention. Role of the ICPC. Available at: osap.org/?page= RoleofICPC. Accessed September 1, 2016.
From Dimensions of Dental Hygiene. October 2016;14(10):40-43.





