
The New Era of Periodontal Regeneration
This course was developed in part with an unrestricted educational grant from Colgate Palmolive Company and in collaboration with the American Academy of Periodontology.
This course was published in the July 2011 issue and expires July 2014. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Discuss current technology for the regeneration of the periodontium, including hard and soft tissues.
- Explain the use of growth factors in periodontal regeneration.
- Describe the use of live cell therapies in soft tissue regeneration.
Introduction
The Colgate-Palmolive Company is delighted to have provided an unrestricted educational grant to support the third article in this CE series developed in collaboration with the American Academy of Periodontology. Tissue engineering has undergone significant transformation in recent years. This article provides a valuable review of the technologies available for use today as well as insights into potential future innovations. We hope that you find this article interesting and of benefit to both your clinical practice and your patients.
—Barbara Shearer, BDS, MDS, PhD, Associate Director of Scientific Affairs, Colgate Oral Pharmaceuticals
Over the past several years, dentistry has experienced an explosion in technological advancement. Specifically, periodontology has seen much innovation with tissue engineering-based regeneration. Today, periodontists and dental hygienists can predictably change the prognoses of teeth by controlling oral disease and reconstructing lost hard and soft tissues with fascinating and powerful new technologies.
The primary goal of periodontal treatment is the maintenance of health, esthetics, and function in the natural dentition and supporting structures. When necessary, this includes tooth and tissue replacement with regeneration and dental implants. Table 1 includes some of the technological advances in periodontal and implant site reconstructive care.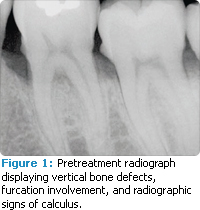
Periodontists have been practicing tissue engineering for decades, beginning with guided tissue regeneration, a form of passive tissue engineering that uses barrier membranes to channel new bone and tissue growth to the sites that need it.1,2 Today the dental team has the ability to introduce biologic therapies, such as growth factors and morphogens, to actively influence wound repair.
Tissue engineering continues to evolve but there are limitations on how much wound healing can be influenced. Introducing the correct growth factor—at the right time in an effective concentration and for the proper duration—is the ultimate goal. Just as we strive to understand the host/systemic role in the pathogenesis of periodontal diseases, we must also take into consideration the host influence, both positive and negative, on wound healing following regenerative procedures.
Procedures that avoid the need for autogenous tissue have motivated surgeons to search for predictable, efficient strategies that use engineered tissue, growth factors, and biomimetic therapies. Tissue engineering is used in many areas of medicine and some of these have crossed over to dentistry.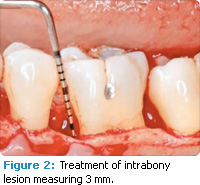
Periodontal regeneration includes the periodontal ligament, cementum and alveolar bone, and soft tissue along the root surface and in interdental spaces, commonly referred to as “black triangles.” The evidence supporting guided tissue regeneration is vast and growing. For years dental surgeons have been using—with high levels of predictability—resorbable and nonresorbable barrier membranes in passive tissue engineering around teeth and dental implant dehiscence and fenestrations.3,4 By employing active tissue engineering, new levels of predictability and success can be reached to resolve hard tissue and soft tissue periodontal defects.
BIOLOGICS AND GROWTH FACTORS
Periodontal defects include the intrabony and furcation-type defects along with gingival recession or loss of attachment. The periodontal literature validates the regenerative potential of biologically active bone replacement grafts (Figures 1-5).5,6 Periodontal regeneration with histological evidence of new cementum, periodontal ligament, and alveolar bone has been shown in the human model with a recombinant growth factor and a porcine (pig)-derived protein.7-9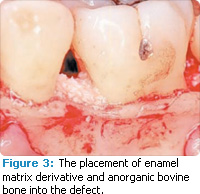
The ability to reconstruct the tissues in an area that was previously infected by periodontitis serves as the ultimate regenerative test. Human recombinant platelet-derived growth factor (PDGF-BB), a protein that regulates cell growth and division, and beta tricalcium phosphate (?-TCP), a drug delivery system for bone, have been shown to accelerate clinical attachment level gains and significantly increase bone growth in severe periodontal defects.10 Recombinant human platelet-derived growth factor (rhPDGF-BB) and enamel matrix derivative (EMD) have demonstrated tissue regeneration when used adjacent to a previously diseased root surface.7-9+
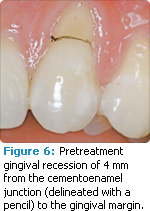
Histologic evidence from a recent study shows that regeneration can be achieved with rhPDGF-BB, ?-TCP, and collagen in gingival recession-type defects.11 Gingival recession defects have been treated successfully with rhPDGF, ?-TCP, and a collagen membrane with a root coverage technique (Figures 6-7). This technique was compared to a subepithelial connective tissue graft within a case series,12 and more recently in a controlled clinical trial. The comparisons demonstrated that the growth factor technique was comparable to the others in root coverage outcomes.13 Many studies validate the use of EMD for periodontal and mucogingival defects.5,6,14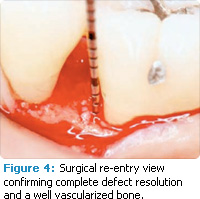
During clinical regenerative therapies, maintaining adequate blood supply and providing appropriate scaffolding for new tissue formation are key.15 The successful use of recombinant growth factors depends on ensuring that the growth factor is present at the correct concentration and volume. It must then communicate properly with host cells for the appropriate amount of time and work with the host feedback mechanisms to alter activity during the different stages of wound healing. The optimal matrices needed to carry and maintain these growth factors in the wound—for the appropriate time and in efficacious concentrations—must be determined through additional wound kinetic studies. The use of human recombinant growth factors has a promising future for reconstructive therapy in both periodontics and implant dentistry by influencing the early stages of wound healing.
TABLE 1. OUTLINE OF TISSUE ENGINEERING AND CELL THERAPY FOR PERIODONTAL THERAPY FOR PERIODONTAL REGENERATIVE PROCEDURES.
Passive
- Guided tissue regeneration-based therapies
- Biologically based-acellular dermal grafts from a human donor
Active
- Platelet-rich plasma
- Enamel matrix derivative
- Growth factors and biological mediators: rh-PDGF-BB, BMP-2 &7, GDF-5, FGF
- Cell therapy
1. Autologous fibroblast (patient biopsy)
2. Bilayered cell therapy (fibroblast human donor)
3. Human fibroblast-derived dermal substitute (fibroblast human donor)
LIVE CELL-BASED THERAPIES
Effective augmentation techniques to treat more challenging esthetic concerns, such as open interproximal spaces and other severe oral soft-tissue deficiencies, are not currently available. Cell-based therapies may change this. Live cell-based therapy delivers multiple growth factors and can communicate more dynamically with host cells.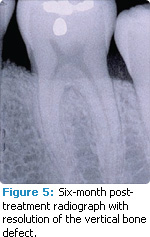
This facet of oral soft-tissue engineering involves the implantation of live cell-based skin substitutes or the in vitro construction of an autologous, transplantable vital tissue. Historically, one of the first bioengineered skin substitutes consisted of autologous expanded fibroblast grown from the patient’s own biopsies. One of the first dental studies using this autologous fibroblast transplantation evaluated the ability to expand interdental gingival soft tissue deficiencies. Results showed the injection of autologous fibroblasts created significant improvement when compared to a placebo. Prior to the injection of these expanded fibroblasts, a “priming” procedure was performed in the papillary region to create an inflammatory response. The theory was to develop temporary increased tissue volume by causing inflammation, thus enabling the injection of more volume of the concentrated cell suspension.
The cells were delivered 5 days to 7 days after the priming procedure and then again at two other times following the first injection. The subjects were assessed at 2 months, 3 months, and 4 months after the injections. Preliminary results indicate a novel tissue-engineering technique may hold promise in resolving the challenging open interproximal space.16 Further research is under way to evaluate this as a predictable therapy.
Other studies are being conducted on the use of dermal substitutes containing fibroblasts to treat gingival recession and attachment loss defects where no attached gingiva is present. Although results are not yet available, the technology holds promise in developing new attached gingiva when compared to the free autogenous graft.17
 |
 |
A study was conducted to evaluate the safety and efficacy of another live cell material, bilayered cell therapy (BCT). This was compared to a free gingival graft in generating attached and keratinized gingiva around teeth not requiring root coverage.18 To date, preliminary periodontal results appear encouraging and, based on these early findings, a large multicenter trial will soon be published revealing the clinical efficacy of this live cell technology.19 These therapies need to be validated with human histology and other well-controlled, randomized clinical trials.
FUTURE HOLDS PROMISE
New technologies will allow dental professionals to more predictably restore lost tissues around teeth and dental implants that have suffered tissue losses from disease, trauma, pathology, and/or treatment failure. Further investigation with recombinant growth factors and live cell therapy needs to be conducted to support these methods for everyday clinical practice.
Implanting live cells is a new dimension of treatment. It is hoped that the live cells will communicate with native cells, optimizing the influx of metabolically active molecules at the appropriate time and in the quantity required by the wound. It will not be long before live cell technologies are available in the dental office. These technologies not only deliver growth factors but also provide a template for cell migration, adhesion, proliferation, and differentiation, thus optimizing the site-specific regenerative response.
During the next decade, the dental team will be able to rewrite some of the rules of regeneration, providing therapies with fewer side effects, shorter treatment times, and optimal predictability. As we continue our quest for the ultimate regenerative response, there is no doubt that tissue engineering will play a profound role in the future, providing tomorrow’s clinician with bio-active materials that strongly influence the treatment outcome for the dental team and, most importantly, the patient.
GLOSSARY OF TERMS
Autogenous: Originating within the body.
Autologous: Originating from the patient (ie, one individual is both donor and recipient).
Barrier membrane: Material used to keep the epithelium from growing into space in the oral cavity that is reserved for bone during the process of guided tissue regeneration.
Biomimetic therapies: Therapies that imitate nature; in tissue engineering, they incorporate cell-binding peptides into biomaterials through chemical or physical modifications.
Dental implant dehiscence: A break in the epithelium that leaves the implant surface from the top of the implant head to the point where it is covered by bone exposed to the oral cavity.
Dental implant fenestration: Exposed implant surface caused by insufficient buccolingual alveolar bone width or poor implant placement.
Growth factors: Proteins that encourage cell growth, proliferation, and differentiation.
Morphogens: Substances that direct the development of tissue.
Recombinant growth factor: Recombined genetic material (DNA) that produces a protein, which provides biologic activity.
Wound kinetics: Complicated mechanisms of wound repair.
The views expressed in this article are those of the authors, not necessarily those of the Colgate-Palmolive Company.
REFERENCES
- Nyman S, Lindhe J, Karring T, Rylander H. New attachment following surgical treatment of human periodontal disease. J Clin Periodontol. 1982;9:290-296.
- Gottlow J, Nyman S, Lindhe J, Karring T, Wennstrom J. New attachment formation in the human periodontium by guided tissue regeneration. J Clin Periodontol. 1986;13:604-616.
- Cortellini P, Clauser C, Pini-Prato GP. Histologic assessment of new attachment following the treatment of a human buccal recession by means of a guided tissue regeneration procedure. J Periodontol. 1993;64:387-391.
- Mellonig JT, Nevins M. Guided bone regeneration of bone defects associated with implants: an evidence based outcome assessment. Int J Periodontics Restorative. Dent 1996;15:169-182.
- Scheyer ET, Velasquez-Plata D, Brunsvold MA, Lasho DJ, Mellonig JT. A clinical comparison of a bovine-derived xenograft used alone and in combination with enamel matrix derivative for the treatment of periodontal osseous defects in humans. J Periodontol. 2002;73:423-432.
- Velasquez-Plata D, Scheyer ET, Mellonig JT. Clinical comparison of an enamel matrix derivative used alone or in combination with a bovine-derived xenograft for the treatment of periodontal osseous defects in humans. J Periodontol. 2002;73:433-440.
- Camelo M, Nevins ML, Schenk RK, Lynch S, Nevins M. Periodontal regeneration in human class II furcations using purified recombinant human platelet-derived growth factor-BB (rhPDGFBB) with bone allograft. Int J Periodontics Restorative Dent. 2003;23:213-225.
- Nevins M, Camelo M, Nevins ML, Schenk RK, Lynch SE. Periodontal regeneration in humans using recombinant human platelet derived growth factor-BB (rhPDGF-BB) and allogeneic bone. J Periodontol. 2003;74:1282-1292.
- Yukna RA, Mellonig JT. Histologic evaluation of periodontal healing in humans following regenerative therapy with enamel matrix derivative. A 10-case series. J Periodontol. 2000;71:752-759.
- Nevins M, Giannobile WV, McGuire MK, et al. Platelet-derived growth factor stimulates bone fill and rate of attachment level gain: Resuults of a large multicenter randomized controlled trial. J Periodontol. 2005;76:2205-2215.
- McGuire MK, Scheyer ET,Nevins M, Schupbach P. Evaluation of human recession defects treated with coronally advanced flaps and either purified recombinant human platelete-derived growth factor (rhPDGF) with beta tricalcium phosphate (?-TCP) or connective tissue: A histological and micro-CT examination. Int J Perio Rest Dent. 2009;29:6-21.
- McGuire MK, Scheyer ET. Comparison of rhPDGF-BB plus beta tricalcium phosphate and a collagen membrane to subepithelial connective tissue grafting for the treatment of recession defects: a case series. Int J Periodontics Restorative Dent. 2006;26:127-133.
- McGuire MK, Scheyer ET. Growth factor mediated treatment of recession defects: A randomized controlled clinical trial and histological and micro-CT examination. J Periodontol. 2009;80:550-564.
- McGuire MK, Nunn M. Evaluation of human recession defects treated with coronally advanced flaps and either enamel matrix derivative or connective tissue. Part 1: Comparison of clinical parameters. J Periodontol. 2003;74:1110-1125.
- Cochran DL, Wozney JM. Biological mediators for periodontal regeneration. Periodontol 2000. 1999;19:40-58.
- McGuire MK, Scheyer ET. A randomized, double-blind, placebo controlled study to determine the safety and efficacy of cultured and expanded autologous fibroblast injections for the treatment of interdental papillary insufficiency associated with the papilla priming procedure. J Periodontol. 2007;78:4-17.
- McGuire MK, Nunn M. Evaluation of human recession defects treated with coronally advanced flaps and either enamel matrix derivative or connective tissue. I: Comparison of clinical parameters. J Periodontol. 2003;74:1110-1125.
- McGuire MK, Scheyer ET, Nunn ME, Lavin PT. A pilot study to evaluate a tissue engineered bi-layered cell therapy as an alternative to tissue from the palate. J Periodontol. 2008;79:1847-1856.
- McGuire MK, Scheyer ET, Nevins M, Giannobile W, Cochran DL, Mellonig JT. Living cellular construct for increasing the width of keratinized gingiva. Results from a randomized, within-patient, controlled trial. J Periodontol. In press.
From Dimensions of Dental Hygiene. July 2011; 9(7) Insert.



