
The Future Looks Bright
How plasma medicine may change the face of dental practice.
This course was published in the December 2011 issue and expires December 2014. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Define low temperature atmospheric pressure plasma (LTAPP).
- Discuss the possible role of LTAPP in sterilization and tooth whitening.
- Identify LTAPP’s ability to inhibit caries-causing bacteria and periodontal pathogens.
- Explain LTAPP’s future applications in dentistry.
Plasma medicine refers to a new field of multidisciplinary research involving the biomedical applications of plasma—an ionized gas. Since the 1990s, researchers have been investigating novel ways to use low temperature atmospheric pressure plasma (LTAPP) to inactivate a broad spectrum of microorganisms and accelerate wound healing.1-4 Recent discoveries in plasma science include treating skin disorders such as diabetic ulcers; eradicating tumors; and cosmetic skin rejuvenation.5-7 LTAPP applications are now making a lateral transition to dentistry for use in sterilization and disinfection, inactivation of oral pathogens, and enhanced tooth whitening.
Low Temperature Atmospheric Pressure Plasma
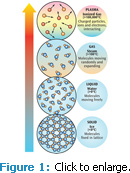
The universe contains four states of matter: solid, liquid, gas, and plasma (Figure 1). Plasma occurs naturally and comprises 99% of the visible world. Plasma is a gas mixture of charged ions and electrons that are activated by energy, such as electricity. Plasma is found in the sun, lightening, neon and fluorescent light bulbs, and televisions. There are two categories of plasma: thermal and nonthermal. Thermal plasmas are hot to the touch and require pressure and a significant power source to operate. Nonthermal plasma is artificially generated using ambient air, and it requires much less power to function. LTAPP is particularly relevant to medicine and dentistry because it is simple to manipulate and can be applied directly to the skin without causing cellular damage.8,9
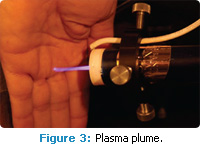
 Several devices have been designed for medical and dental applications. The LTAPP plasma pencil (Figure 2), developed by Laroussi et al, is a handheld device that resembles a miniature lightsaber. It emits a plasma plume that can deliver direct nonthermal plasma to a target, and remain stable for several hours without damaging human tissue (Figure 3).9 LTAPP is effective in the inactivation of numerous pathogenic microorganisms, including: Geobacillus stearothermophilus, Bacillus cereus, Streptococcus mutans, Porphyromonas gingivalis, Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, and Aspergillus niger.10-14 Although the mechanism of action is not clearly understood, research suggests that LTAPP exposure either disturbs or destroys cell membranes or renders cells unable to reproduce.
Several devices have been designed for medical and dental applications. The LTAPP plasma pencil (Figure 2), developed by Laroussi et al, is a handheld device that resembles a miniature lightsaber. It emits a plasma plume that can deliver direct nonthermal plasma to a target, and remain stable for several hours without damaging human tissue (Figure 3).9 LTAPP is effective in the inactivation of numerous pathogenic microorganisms, including: Geobacillus stearothermophilus, Bacillus cereus, Streptococcus mutans, Porphyromonas gingivalis, Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, and Aspergillus niger.10-14 Although the mechanism of action is not clearly understood, research suggests that LTAPP exposure either disturbs or destroys cell membranes or renders cells unable to reproduce.
Sterilization
Current sterilization methods in dentistry include steam, dry heat, and the use of chemicals, such as ethylene oxide. These techniques are slow, energy inefficient, and can be toxic to humans and the environment. In some instances, repeated use of heat and chemicals can damage instruments and equipment. As a result, other sterilization methods are in demand, and LTAPP may provide such an alternative.
The gold standard in evaluating the effectiveness of heat sterilization is the use of biological indicator test strips containing G. stearothermophilus and B. cereus. Both microbes are heat resistant at the spore stage.15,16 B. cereus is often associated with food poisoning, and G. stearothermophilus is an appropriate yardstick for measuring bacterial destruction. When LTAPP was used on both organisms, the vegetative states of each organism and the spore state of B. cereus were completely destroyed. Spores of G. stearothermophilus, however, were not completely eliminated.16 Modifications of the cold plasma device to improve its success in G. stearothermophilus spore destruction are ongoing.
Caries
The prevalence of S. mutans is evident in nearly all populations across the globe,17 and caries remains one of the most common chronic, transmittable, destructive—yet preventable— diseases. Contemporary caries prevention methods include the use of fluorides and mechanical biofilm removal, but optimal plaque control is not always achieved. Restorative treatment requires removing the infected tooth structure, as well as healthy tooth structure, and replacing lost structure with metal or composite. A more advantageous proposal for the management of dental caries may be to destroy the key causative agent—S. mutans—leaving healthy tissue intact. Several studies have shown that nonthermal plasma has the ability to destroy or inactivate S. mutans.18-22 A recent study found that the plasma pencil inactivated S. mutans in as little as 60 seconds.20 The application of LTAPP to S. mutans for various intervals demonstrated significant bacterial inactivation.18-22 Given that LTAPP has a time/dose response effect and is capable of penetrating small areas, applying it to enamel pits and fissures may reduce ccaries-causing microorganisms.
Periodontal Pathogens
Periodontal diseases are chronic oral infections caused by several anaerobic periodontal pathogens, including P. gingivalis. Research has found that periodontal diseases affect overall systemic health, making their effective treatment imperative.23 Periodontal diseases are traditionally treated with the removal of pathogens through scaling and root planing and locally or systemically delivered chemotherapeutic agents.24 Neither of these methods sustains long-term control of periodontal pathogens, and they may not reach the base of deep pockets. A recent study suggests that LTAPP is capable of inactivating P. gingivalis. Results showed that the ability of LTAPP to inhibit bacteria increased the longer it was employed.25 Because LTAPP can be targeted in a confined space, it may have potential as a site-specific treatment to inactive periodontal pathogens and accelerate wound healing.
Tooth Whitening
Tooth whitening is one of the most requested dental procedures—most patients want whiter teeth and a more esthetic smile. Yet, whitening procedures are time consuming and often cause dentinal hypersensitivity and mucosal irritation. Typically, in-office tooth whitening procedures require the use of relatively high percentages of hydrogen peroxide, ranging from 25% to 35%, and some type of accelerator light. In an effort to develop alternative methods of tooth whitening, researchers are studying the effects of LTAPP as a light source to augment the whitening process (Figure 4). Literature supports the theory that tooth whitening is enhanced by using LTAPP with a peroxide gel, compared to using peroxide alone.26–30
 During the whitening process, hydrogen peroxide breaks down to produce hydroxyl radicals. Accelerant lights speed up this oxidation process, which ultimately reacts with organic and inorganic compounds and leads to whitening. Although the plasma-whitening relationship is not well understood, researchers theorize that LTAPP enhances the production of reactive oxygen hydrogen radicals.
During the whitening process, hydrogen peroxide breaks down to produce hydroxyl radicals. Accelerant lights speed up this oxidation process, which ultimately reacts with organic and inorganic compounds and leads to whitening. Although the plasma-whitening relationship is not well understood, researchers theorize that LTAPP enhances the production of reactive oxygen hydrogen radicals.
Studies show that the amount of oxygen hydrogen generated from hydrogen peroxide improves whitening after various time exposures,26 and the use of plasma with hydrogen peroxide increases the production of reactive oxygen hydrogen radicals.29 When compared to using hydrogen peroxide alone, plasma was more successful in lightening coffee and red wine stains in extracted teeth.29 Additional research shows that plasma-enhanced whitening occurs without thermally damaging the tooth or altering morphology or microhardness.29 In a recent study that used the LTAPP pencil, whitening occurred in as little as 10 minutes.30 LTAPP’s role in tooth whitening has yet to be defined, but foundational studies suggest it can safely enhance the tooth-whitening process.
Future Research
LTAPP is currently being investigated for use in endodontic therapy.31 Generally, root canal disinfection is accomplished through the use of sodium hypochlorite and other antimicrobial agents. Researchers are experimenting with plasma as a root canal disinfectant because of its ability to navigate cracks, crevices, and convoluted areas. If LTAPP applications are successful, it could reduce failed endodontic treatment.
LTAPP has proven successful as a surface modification agent in other industries, such as food safety. Food packages are treated with plasma in an effort to reduce pathogenic microorganisms from adhering and proliferating in containers.32 The interactions of plasma-treated surfaces are complex, yet research has shown that areas treated with LTAPP prevent bacterial adherence and microbial colonization.32 In dentistry, the application of a plasma layer to an implant may inhibit biofilm adherence and growth that could contribute to reducing periimplant disease.33 Additionally, treating dental materials, enamel, or dentin surfaces with LTAPP may enhance adhesion for certain types of restorations.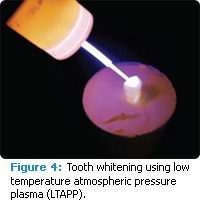
Plasma has demonstrated success in wound and tissue healing, hence the leap to treating oral lesions is a natural one. Plasma may assist the curative process by inactivating pathogenic microorganisms while leaving healthy cells intact. In dentistry, common oral diseases, such as candidiasis, apthous ulcers, herpetic lesions, as well as infected extraction and periodontal sites, may benefit from the therapeutic effects of LTAPP. Employing plasma to combat opportunistic infections would be an alternative to present-day mechanical, chemical, and antimicrobial management.
Conclusion
The future of plasma medicine is promising and the potential is tangible. Plasma applications range from water treatment, sterilization and disinfection, inactivation of environmental and oral pathogens, wound healing, surface modification, and tooth whitening. Plasma science represents a shift away from chemical and mechanical therapies to those that are molecular based. The cooperation between specialists in medicine, dentistry, engineering, chemistry, physics, and microbiology has created an innovative specialty. Proof-of-concept research demonstrates the ability of plasma’s biomedical applications to segue into dentistry.
Acknowledgement
The authors would like to thank Mounir Laroussi, PhD, director of the Laser and Plasma Engineering Institute at Old Dominion University, for his expertise and assistance with plasma research.
PHOTO CREDITS
FIGURE 1. COURTESY OF GAYLE B. MCCOMBS, RDH, MS, AND DONALD K. EMMINGER
FIGURE 2. CHARLES E. THOMAS
FIGURE 3. CHARLES E. THOMAS
REFERENCES
- Laroussi M. Sterilization of contaminated matter with an atmospheric pressure plasma. IEEE Trans Plasma Sci IEEE Nucl Plasma Sci Soc. 1996;24:1188–1191.
- Kalghatgi S, Fridman G, Balasubramanian M, et al. Mechanism of blood coagulation by nonthermal atmospheric pressure dielectric barrier discharge. Paper presented at: IEEE Pulsed Power Plasma Science Conference; June 17, 2007; Albuquerque, NM.
- Kalghatgi SU, Fridman A, Friedman G, Clyne AM. Nonthermal plasma enhances endothelial cell proliferation through fibroblast growth factor-2 release. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:3578–3581.
- Morfill GE, Shimizu T, Steffes B, Schmidt HU. Nosocomial infections—new approach towards preventive medicine using plasmas. N J Phys. 2009;11:115019.
- Bogaerts A, Neyts E, Gijbels R, Van Der Mullen J. Gas discharge plasmas and their applications. Spectrochim Acta A Mol Biomol Spectrosc. 2002;57:609–658.
- Foster KW, Moy RL, Fincher EF. Advances in plasma skin regeneration. J Cosmet Dermatol. 2008;7:169–179.
- Kilmer S, Semchyshyn N, Shah G, Fitzpatrick R. A pilot study on the use of a plasma skin regeneration device (Portrait PSR3) in full facial rejuvenation procedures. Lasers Med Sci. 2007;22:101–109.
- Laroussi M. The biomedical applications of plasma: a brief history of the development of a new field of research. IEEE Trans Plasma Sci IEEE Nucl Plasma Sci Soc. 2008;36:1612–1614.
- Lu X, Tendero C, Laroussi M. The plasma pencil: a novel cold plasma source for low temperature applications. Paper presented at: International Conference on Plasma Science; June 4, 2006; Traverse City, Mich.
- Laroussi M, Minayeva O, Dobbs F, Woods J. Spores survivability after exposure to lowtemperature plasmas plasma science. IEEE Trans Plasma Sci IEEE Nucl Plasma Sci Soc. 2006;34:1253–1256.
- Laroussi M, Richardson P, Dobbs F. Effects of nonequilibrium atmospheric pressure plasmas on the heterotrophic pathways of bacteria and on their cell morphology. Appl Phys Lett. 2002;81:1409–1415.
- Kelly-Wintenberg K, Hodge A, Monite T, et al. Use of a one atmosphere uniform glow discharge plasma to kill a broad spectrum of microorganisms. J Vac Sci Technol. 1999;17:1539–1544.
- Abramzon N, Joaquin J, Bray J, Brelles-Marino G. Biofilm destruction by RF high-pressure cold plasma jet. IEEE Trans Plasma Sci IEEE Nucl Plasma Sci Soc. 2006;34:1304–1309.
- Richardson J, Dyer F, Dobbs F, Alexeff I, Laroussi M. On the use of the resistive barrier discharge to kill bacteria: Recent results. Proc IEEE Int Conf Plasma Sci. 2000:109.
- Schneider PM, Reich RR, Kirckof SS, Foltz WG. Performance of various steam sterilization indicators under optimum and sub-optimum exposure conditions. Am J Infect Control. 2005;33(Suppl):S55–S67.
- Morris A, McCombs G, Tolle S, Laroussi M, Hynes W. Bactericidal effects of cold plasma technology on Geobacillus stearothermophilus and Bacillus cereus microorganisms. J Dent Hyg. 2009;83:55–61.
- US Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. Atlanta: US Department of Health and Human Services; 2005.
- Goree J, Bin L, Drake D, Stoffels E. Killing of S. mutans bacteria using a plasma needle at atmospheric pressure. IEEE Trans Plasma Sci IEEE Nucl Plasma Sci Soc. 2006;34:1317–1324.
- Goree J, Bin L, Drake D, Stoffels E. Disinfection of S. mutans bacteria using a plasma needle at atmospheric pressure. Paper presented at: IEEE International Conference on Plasma Science; June 4, 2006; Piscataway, NJ.
- Lemaster M, McCombs G, Darby M, Hynes W, Laroussi M. The effects of low temperature atmospheric pressure plasma on Streptococcus mutans [thesis]. Norfolk, Va: Old Dominion University; 2009.
- Sladek R, Baede T, Stoffels E. Plasma-needle treatment of substrates with respect to wettability and growth of Escherichia coli and Streptococcus mutans. IEEE Trans Plasma Sci IEEE Nucl Plasma Sci Soc. 2006;34:1325–1330.
- Sladek R, Filoche S, Sissons C, Stoffels E. Treatment of Streptococcus mutans biofilms with a nonthermal atmospheric plasma. Lett Appl Microbiol. 2007;45:318–323.
- Kuo L-C, Polson AM, Kang T. Associations between periodontal diseases and systemic diseases: A review of the inter-relationships and interactions with diabetes, respiratory diseases, cardiovascular diseases and osteoporosis. Public Health. 2008;122:417–433.
- Carranza F, Newman M, Takei H, Klokkevold P. Carranza’s Clinical Periodontology. St. Louis: Saunders Elsevier; 2006.
- Mahasneh A, Darby M, Tolle L, Laroussi M, Hynes W. Bactericidal Effects of Low Temperature Atmospheric Pressure Plasma on Porphormonas gingivalis, [thesis]. Norfolk, Va: Old Dominion University; 2009.
- Lee HW, Kim GJ, Kim JM, Park JK, Lee JK, Kim GC. Tooth bleaching with nonthermal atmospheric pressure plasma. J Endo. 2009;35:587–591.
- Lee HW, Nam SH, Mohamed A-AH, Kim GC, Lee JK. Atmospheric pressure plasma jet composed of three electrodes: application to tooth bleaching. Plasma Process Polym. 2010;7:274–280.
- Pan J, Sun P, Tian Y, et al. A novel method of tooth whitening using cold plasma microjet driven by direct current in atmospheric-pressure air. IEEE Trans Plasma Sci IEEE Nucl Plasma Sci Soc. 2010;38:3143–3151.
- Sun P, Jie P, Ye T, et al. Tooth whitening with hydrogen peroxide assisted by a direct-current cold atmospheric-pressure air plasma microjet. IEEE Trans Plasma Sci IEEE Nucl Plasma Sci Soc. 2010;38:1892–1896.
- Claiborne D, McCombs G, Lemaster M, Laroussi M. Effects of low temperature atmosphere pressure plasma on tooth whitening [thesis]. Norfolk, Va: Old Dominion University; 2011.
- Chunqi J, Meng-Tse C, Schaudinn C, et al. Pulsed atmospheric-pressure cold plasma for endodontic disinfection. IEEE Trans Plasma Sci IEEE Nucl Plasma Sci Soc. 2009;37:1190–1195.
- Wan J, Coventry J, Swiergon P, Sanguansri P, Versteeg C. Advances in innovative processing technologies for microbial inactivation and enhancement of food safety—pulsed electric field and low-temperature plasma. Trends in Food Science & Technology. 2009;20:414–424.
- Yoshinari M, Matsuzaka K, Inoue T. Surface modification by cold-plasma technique for dental implants—bio-functionalization with binding pharmaceuticals. Jpn Dent Sci Rev. 2011;47:89–101.
From Dimensions of Dental Hygiene. December 2011; 9(12): 40-43.



