
The Dynamic Process of Demineralization and Remineralization
The remineralization of incipient carious lesions as an alternative to conventional caries treatment.
Dental caries is a complex, multifactorial, transmittable infectious disease caused by the process of demineralization and remineralization in the presence of fermentable dietary carbohydrates, saliva, and cariogenic oral flora. The disease continues to be highly prevalent in the United States and other countries around the world. The 2001 Report of the Surgeon General—Oral Health In America— stated that 7% of children aged 2 years to 17 years had unmet dental needs.1 A 2006 survey found that 50% of children aged 5 years to 9 years had at least one cavity or filling with this proportion increasing to 78% among 17-year-olds.2
Shortly after the teeth erupt into the mouth, a protective layer of saliva-derived proteins— the acquired enamel pellicle (AEP)— forms on the tooth. A sticky, tenacious, and highly complex biofilm is created when dental plaque forms on the AEP and oral flora colonize it. The process of demineralization and dental caries formation begins when cariogenic microorganisms are present in large numbers and dietary fermentable carbohydrates become available in the dental biofilm.3 A white spot lesion initially appears. If demineralization continues, it results in cavitation of the tooth.
Many oral microorganisms are capable of forming organic acids that reduce the pH of the dental plaque when exposed to carbohydrates. Numerous streptococcus strains, including S. mutans, S. sanguinis, and to a lesser extent, lactobacillus, are considered important bacteria involved in the development of dental caries. However, our knowledge of the initial colonization of the oral biofilm, its maturation, and the microbial mediated caries processes remains incomplete. These organisms colonize the oral cavity prior to or shortly after the eruption of the first tooth.
The infant’s oral cavity is often infected with S. mutans by transmission from a caregiver, usually the mother. Children colonized by S. mutans by the age of 2 years are much more likely to experience early childhood caries than children lacking cultivable S. mutans.3-5
Dental enamel is composed primarily of hydroxyapatite with smaller amounts of water, protein, and trace elements including fluoride. The enamel of newly erupted teeth is less dense and more permeable and soluble than mature enamel. The AEP assists in the posteruptive maturation of the dental enamel, considerably reducing its porosity. The application of topical fluoride to newly erupted teeth can also significantly increase caries resistance.6,7
THE PROCESS
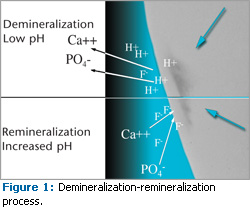
The mineral composition and structure of the enamel surface are partially products of the dynamic demineralization and remineralization process. The dental biofilm modulates the tooth surface pH impacting this process. Dietary ingestion of fermentable carbohydrates, especially sucrose, provides the substrate for the cariogenic microorganisms in the biofilm to form organic acids. The enamel demineralization process begins when these acids lower the pH of the biofilm to below 5.5. The acids result in the loss of calcium and phosphates from the surface and subsurface enamel into the AEP and biofilm, creating a white spot lesion. A white spot lesion is characterized by low calcium and phosphate content and is the initial detectable evidence of enamel demineralization in the subsurface region of the tooth. The white spot lesion will progress into frank cavitation if the bacterial plaque is not regularly removed from the tooth surface.8
The demineralization process is reversible provided that the acidogenic properties of the biofilm are neutralized. The buffering capacity of saliva plays a critical role in helping restore a neutral pH at the tooth surface. Remineralization occurs when the dietary carbohydrate is removed and the pH of the biofilm is raised to approximately 7.0. Once the pH returns to higher than the critical point, demineralization is arrested and minerals can be added back to the partially dissolved enamel crystallites.
Treatment of early caries by remineralization has the potential to significantly advance noninvasive clinical management of the disease. Calcium and phosphate in the saliva and plaque permit the recovery of some lost mineral content by the enamel. Extremely high calcium and phosphate concentrations in the dental pellicle can actually adversely affect the quality of re mineralization.9 High concentrations favor formation of calcium-phosphate mineral phases on the surface that occlude the enamel pores and limit remineralization of the subsurface enamel. A more complete remineralization process occurs when the calcium and phosphate are lower.3,10 Laboratory research suggests that caries extending into the dentin can also be remineralized.11
FLUORIDE
Fluoride is the most reactive element in the periodic table and its presence in the biofilm is important in limiting demineralization and stimulating remineralization of the hydroxyapatite crystal. Fluoride ions react with the partially dissolved enamel crystallites and attract calcium and phosphate ions in the saliva to the demineralized dental enamel. This enhances new mineral deposition and crystallite re-growth. In addition, the presence of fluoride favors the formation of enamel fluorapatite by substitution of hydroxyl molecules with fluoride in the hydroxyapatite crystal. Fluorapatite is harder and more resistant to acid dissolution than hydroxyapatite.
Frequent exposure of the teeth to low concentrations of fluoride is thought to produce the optimal remineralization environment. This can be achieved by the regular ingestion of fluoridated drinking water due to its topical effects, daily use of over-the-counter oral rinses containing 0.05% sodium fluoride, fluoride-containing chewing gum, and the regular use of fluoride containing dentifrice.12
Periodic, professionally-applied topical fluoride agents can also be beneficial. The American Dental Association has approved the use of 1.23% acidulated phosphate fluoride (APF) gel/foam, 8% stannous fluoride solution, and 2% sodium fluoride gel for professionally applied topical agents.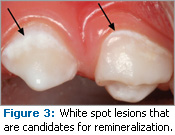
European studies of a 5% sodium fluoride varnish have also demonstrated caries preventive benefits similar to APF gel/foam when applied topically to the teeth.13,14 Because similar studies have not been replicated in the United States, fluoride-containing varnish is only approved for use as a cavity liner and treatment of hypersensitivity by the Food and Drug Administration. However, fluoride-containing varnishes are widely used “off-label” for topical application as caries prevention agents.15
Calcium, phosphate, and fluoride ions in the saliva assist in the remineralization process (Figure 1). Saliva is the vehicle that delivers available fluoride ions to the demineralized enamel and partially dissolved crystallites.16 The predominate enamel/ fluoride reaction products from topical fluoride are CaF2 and CaH(PO4)17 (Figure 2). Without saliva to slowly dissolve the CaF2 and deliver the fluoride ion to the demineralized enamel, the remineralization process will not occur. The topical application of aqueous stannous fluoride solution to the teeth is effective in preventing dental caries but may not encourage the remineralization process as well as other topical fluoride products. This is attributed to the deposition of tin ions from the stannous fluoride on the enamel surface. These ions can occlude the porous enamel thus reducing the bioavailability of the fluoride ions to the demineralized crystallites.
CALCIUM PHOSPHATE
Remineralization may be a noninvasive treatment of early carious lesions and hypomineralized enamel negating the need for invasive dental restorations. The development of products to encourage remineralization is a research goal. Currently, there are three types of remineralization technologies available.
Amorphous calcium phosphate (ACP) is a reactive and soluble calcium phosphate compound that releases calcium and phosphate ions to convert to apatite and remineralize when it comes in contact with saliva. Forming on the tooth enamel within the dentinal tubules, ACP provides a reservoir of calcium and phosphate ions in the saliva.18
Calcium sodium phosphosilicate (NovaMin®) contains calcium, phosphorous, sodium, and silica. It reacts with saliva, releasing Ca2+, P5+, and Na+ into the oral environment. First the Na+ buffers the acid and then the charged Ca2+ and P5 ions saturate saliva precipitating into demineralized areas to form a new layer of hydroxyapatite filling the demineralized lesions.19
Casein phosphopeptides (CPP) is a sticky, milk-derived protein that binds to AEP and bacterial plaque. It stabilizes amorphous calcium phosphate (ACP). Products have recently been introduced containing CPP-ACP or Recaldent™ that use CPP as a vehicle to deliver and maintain a super-saturation state of ACP at or near the tooth surface. Laboratory and limited clinical trials of a professionally- or patient-applied topical CPP-ACP paste show promise in slowing the progression of demineralization and promoting remineralization of white spot lesions20-22 (Figure 3). Some of these products also contain fluoride. Gum, lozenges, and topically applied solutions containing CPP-ACP may also remineralize white spots.23,24
SEALANTS
Sealants are often used to occlude at-risk pits and fissures on teeth. When properly placed, sealants provide a physical barrier between the dental enamel and the oral environment shielding the tooth surface from acid challenge. Sealants are effective in arresting caries progression when properly applied to incipient demineralized lesions.25 Fluoride-releasing sealants are also on the market. The manufacturers of fluoride-releasing sealants claim that their products promote remineralization by releasing fluoride in the immediate area adjacent to the sealant.
CHEWING GUM
The importance of saliva’s buffering capacity in the remineralization of demineralized hard dental tissues and the maintenance of optimum oral health is well established. Recently there has been renewed interest in the benefits of chewing gum as a means to stimulate saliva flow to prevent dental caries.26-28 Contraction of the mastication muscles increases the flow of saliva, resulting in an elevated presence of calcium and phosphate ions, and it raises the pH of the biofilm. All of these traits are important to the remineralization process. Numerous studies have demonstrated the caries-preventing qualities of frequent use of chewing gum sweetened by dietary sugar alcohols such as xylitol and sorbitol.29,30 Chewing gum, particularly sugar-free gum, may offer a valuable adjunct to a caries prevention and remineralization program.
CONCLUSIONS
The caries process is a continuum of many cycles of demineralization and remineralization. Remineralization is the body’s natural process for repairing subsurface non-cavitated carious lesions caused by organic acids created by bacterial metabolism of fermentable carbohydrates. Fluoride ions in the presence of calcium and phosphate promote remineralization by building a new surface on existing crystal remnants in subsurface demineralized lesions. This environment also favors the formation of the more favored fluorapatite crystal in the enamel.
Remineralization of incipient carious lesions is a conservative alternative to conventional caries removal and dental restoration. The development and promotion of a robust caries prevention and remineralization regimen that discourages demineralization and encourages remineralization remains a challenge. Additional research is needed to identify new approaches to stimulate the beneficial effects of the remineralization process, reduce the incidence of dental caries and to optimize health.
REFERENCES
- United States Department of Health and Human Services. Oral Health in America. Available at: www.surgeongeneral.gov/library/oralhealth/. Accessed June 8, 2009.
- Summary Health Statistics for U.S. Children: National Health Interview Survey, 2006. Available at: www.cdc.gov/nchs/data/series/sr_10/sr10_234.pdf. Accessed June 8, 2009.
- Garcia-Godoy F, Hicks MJ. Maintaining the integrity of the enamel surface: the role of dental biofilm, saliva and preventive agents in enamel demineralization and remineralization. J Am Dent Assoc. 2008;139:(Suppl 2):25S-34S.
- Berkowitz RJ, Jones P. Mouth-to-mouth transmission of the bacterium S. mutans between mother and child. Arch Oral Biol. 1988;30:377-379.
- Caufield PW, Cutter GR, Dasanayake AP. Initial acquisition of mutans by infant: evidence for a discrete window of infectivity. J Dent Res. 1993; 72:37-45.
- Featherstone JD. The caries balance: the basis for caries management risk assessment. Oral Health Prev Dent. 2004;2 (Suppl):259-264.
- Hicks J, Garcia-Godoy F, Flaitz C. Biological factors in dental caries: role of remineralization and fluoride in the dynamic process of demineralization and remineralization (part 3). J Clin Pediatr Dent. 2004;28:203-214.
- Silverstone LM, Featherstone MJ, Hicks MJ. Dynamic factors affecting lesion initiation and progression in human dental enamel. Part I. The dynamic nature of enamel caries. Quintessence Int. 1988;19:683-711.
- Hicks J, Garcia-Godoy F, Flaitz C. Biological factors in dental caries enamelstructure and the caries process in the dynamic process of demineralization and remineralization (part 2). J Clin Pediatr Dent. 2004;28:119-124.
- Silverstone LM, Wefel JS, Zimmerman BF, Clarkson BH, Featherstone MJ. Remineralization of natural and artificial lesions in human dental enamel in vitro. Effect of calcium concentration of the calcifying fluid. Caries Res. 1981;15:138-157.
- ten Cate JM. Remineralization of deep enamel dentine caries lesions. Aust Dent J. 2008;53:281-285.
- ten Cate JM, Feathestone JDB. Physicochemical aspects of fluoride-enamel interactions. In: Fejerskov O, Eksrand J, Burt BA, eds. Fluoride in Dentistry. 2nd ed. Copenhagen: Munksgaard;1996:252-272.
- Haugejorden O, Nord A. Caries incidence after topical application of varnishes containing different concentrations of sodium fluoride: 3-year results. Scand J Dent Res. 1991;99:295-300.
- Borutta A, Künzel W, Rübsam F. The caries-protective efficacy of 2 fluoride varnishes in a 2-year controlled clinical trial. Dtsch Zahn Mund Kieferheilkd Zentralbl. 1991; 79: 543-549. German.
- ADA Current Policies 1954-2007. Fluoride and Fluoridation, page 123. Available at: www.ada.org/prof/resources/positions/doc_policies.pdf. Accessed June 9, 2009.
- Hicks J, Flaitz C. Role of remineralizing fluid in in vitro enamel caries formation and progression. Quintessence Int. 2007;38:313-319
- ten Cate JM, Larsen MJ, Pearce EIF, Fejerskov O. Chemical interactions between the tooth and oral fluids. In: Fejerskov O, Kidd EAM, eds. Dental Caries: The Disease and its Clinical Management. Copenhagen: Blackwell Munksgaard, 2003:4-69.
- Chow LC, Takagi S, Vogel GL. Amorphous calcium phosphate: the contention of bone. J Dent Res. 1998;77:6.
- Reynolds EC. Calcium phosphate-based remineralization systems: scientific evidence? Aust Dent J. 2008;53:268-273
- Reynolds EC, Cai F, Cochraqne NJ, Shen P, Walker GD, Morgan MV, Reynolds C. Fluoride and casein phosphopeptide-amorphous calcium phosphate. J Dent Res. 2008;87:344-348.
- Pai D, Bhat SS, Taranath A, Sargod S, Pai VM. Use of laser florescence and scanning electron micro – scope to evaluate remineralization of incipient enamel lesions remineralized by topical application of casein phosphopeptide amorphous calcium phosphate (CPP-aCP) containing cream. J Clin Pediatr Dent. 2008;32:201-206.
- Morgan MV, Adams GG, Bailey DL, Tsao CE, Fischman SL, Reynolds EC. The anticariogenic effect of sugar-free gum containing CPP-ACP nano complexes on approximal caries determined using digital bitewing radiography. Caries Res. 2008; 42: 171-184.
- Cai F, Shen P, Morgan MV, Reynolds EC. Remineralization of enamel subsurface lesions in situ by sugar-free lozenges containing casein phosphopeptide-amorphous calcium phosphate. Aust Dent J. 2003;48:240-243.
- Morgan M, Adams G, Bailey D, Tsao C, Fischman S. The anticariogenic effect of sugar-free gum containing CPP-ACP nanocomplexes on approximal caries determined using digital bitewing radiography. Caries Res. 2008;42:171-184.
- Oong EM, Griffin SO, Kohn WG, Gooch BF, Caufield PW. The effect of dental sealants on bacteria levels in caries lesions: a review of the evidence. J Am Dent Assoc. 2008;139:271-278.
- Birkhed D. Cariologic aspects of xylitol and its use in chewing gum: a review. Acta Odontol Scand. 1994;52:116-127.
- Tanzer JM. Xylitol chewing gum and dental caries. Int Dental J. 1995;45(1Suppl l):65-76.
- Makinen KK. The rocky road of xylitol to its clinical application. J Dent Res. 2000;79:1352-1355.
- Imfeld T. Efficacy of sweeteners and sugar substitutes in caries prevention. Caries Res. 1993; 27(Suppl 1):50-55.
- Scheie AAa, Fejerskov OB. Xylitol in caries prevention: what is the evidence for clinical efficacy? Oral Dis. 1998:4:268-278.
From Dimensions of Dental Hygiene. July 2009; 7(7): 16,18,20–21.


