 BYMANDESIGNS/ISTOCK/GETTY IMAGES PLUS
BYMANDESIGNS/ISTOCK/GETTY IMAGES PLUS
Subgingival Endoscopic Visualization and Treating Peri-Implantitis
The use of the dental endoscope in nonsurgical treatment of peri-implantitis can improve patient outcomes.
According to the American Academy of Implant Dentistry, 3 million people in the United States have implants. This number is expected to grow by 500,000 each year.1 As the number of patients with implants continues to rise, so does the risk for post-placement complications, which can compromise the health and long-term success of implants.
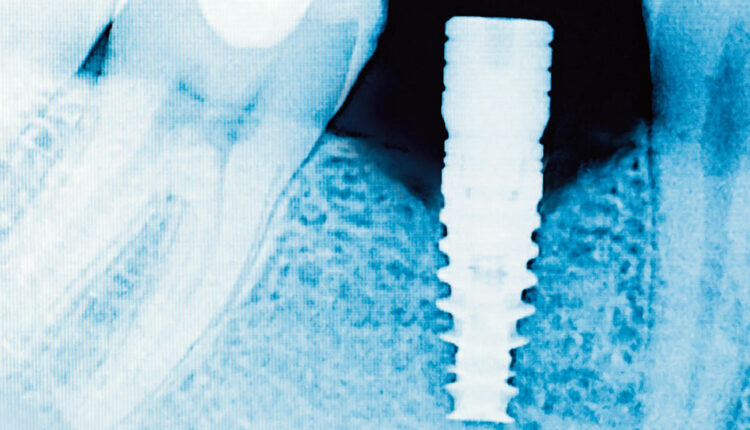
One of the most common implant therapy complications is the destruction of peri-implant gingival tissues and bone by residual cement. In one study, residual cement was associated with 81% of the peri-implantitis cases reviewed.2 This complication is most commonly diagnosed when cement is noted on a radiograph (Figure 1). Residual cement can be found not only on the proximal surfaces but also the buccal and lingual aspects. As traditional radiographs cannot reveal cement on the buccal or lingual surfaces, these cases go unnoticed until clinical signs of infection and peri-implantitis are evident. In addition, cements used to secure the crown have varying degrees of radiopacity. In some cases, cements are completely radiolucent, decreasing the ability to note excess on any surface. To complicate matters, clinical signs of inflammation, including rounded, red gingival margins, bleeding, and exudate may not appear for several years after implant restoration, if at all.3
Periodontal lesions associated with dental implants are histologically larger than the lesions found on natural teeth. In their comparison study, Carcuac and Berglundh4 found periodontal lesions associated with dental implants infiltrated through the connective tissue apical to the pocket epithelium. The lesions associated with natural dentition were limited to the ulcerated lesion evident in the pocket epithelium and surrounded by a margin of unaffected connective tissue. This suggests that left undiagnosed or untreated, the peri-implantitis lesion could result in greater tissue destruction. This correlation is most likely due to the difference in collagen fiber types and insertion points of dental implants vs natural dentition.
SURGICAL APPROACH
In a case where residual cement is suspected as an etiology of peri-implantitis, a traditional periodontal flap procedure may be recommended. This procedure allows the surgeon to visualize the implant surface, remove any residual cement, decontaminate the implant surface, and add bone-forming regenerative materials. However, in some cases, a flap procedure may not be the ideal first step in treatment. Patients who present with a thin gingival biotype or limited keratinized tissue are at risk for post-operative gingival margin changes, including loss of papilla and recession.5 Soft tissue changes could create an unacceptable esthetic result, as well as increase the accumulation of bacterial plaque.6 These post-operative complications are a risk no matter how conservative the surgical approach. Patients may have other barriers to treatment such as fear and cost.
When treating peri-implantitis, scaling and root planing is another option. Dental hygienists are often warned to avoid scratching or altering the implant surface—which when combined with the difficulty inherent to accessing implant surfaces—may discourage clinicians from scaling adequately to remove contaminants such as residual cement.7 Open periodontal flap procedures involving dental implants result in greater reductions in bleeding on probing and pocket depths compared with nonsurgical treatment.8 These results remain true even with the addition of surface decontaminates such as antibiotics and antimicrobial solutions during root planing.8
The advantage of the periodontal flap surgery over traditional scaling and root planing is visualization. The visualization allows the surgeon to address the cement without needless instrumentation on other areas of the implant. This minimizes the potential for surface damage while assuring that all of the cement has been removed. In recent years, the use of dental endoscopes during nonsurgical treatment of natural dentition has become more common. A dental endoscope can magnify a surface by 50 times or more. Research shows that the dental endoscope reduces bleeding on probing and gingival inflammation significantly more than nonvisual, traditional scaling and root planing when used around natural dentition.9 As such, the benefits of the dental endoscope may also extend to the nonsurgical treatment of peri-implantitis. The following case study illustrates the use of the dental endoscope to treat peri-implantitis caused by the presence of residual cement.
CASE STUDY
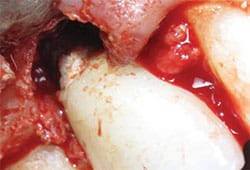
The patient is a healthy, nonsmoking 52-year-old woman with an unimpressive medical history. Tooth #19 had been extracted due to a failing root canal. A dental implant was placed 3 months post-extraction and the patient returned to her restorative dentist to place the implant crown. Five years later, she was referred for evaluation of implant #19, with bleeding and exudate noted on probing. Implant #19 showed no obvious signs of inflammation on initial visual inspection. However, palpating the buccal and lingual aspects yielded exudate. The circumferential probing depths measured 6 mm to 9 mm and there was less than 1 mm of buccal keratinized epithelium. The radiographic exam revealed bone loss to the second implant thread on the distal and the third on the mesial (Figure 2). There was no radiographic evidence of cement.
endoscope visualization.
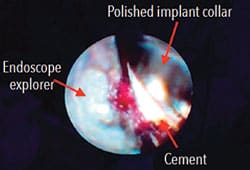
Under local anesthetic, the dental endoscope fiber was placed under the gingival margin and the crown margin and polished implant collar were inspected. Upon inspection, residual cement was noted on the buccal and lingual aspects (Figure 3). An ultrasonic scaler was used to remove the residual cement while simultaneously visualizing the area through the endoscope. This allowed for debridement without needless instrumentation, protecting the implant surface. The implant was carefully debrided until cement was no longer visible through the endoscope.
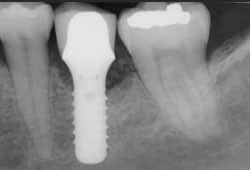
In addition to endoscopic treatment, the surface of the implant was decontaminated using glycine powder, followed by a 5-minute irrigation with 10% iodine solution.10 Finally, a Nd:Yag laser was used to perform a bacterial reduction of the pocket, with caution to prevent soft-tissue curettage.11 The patient returned for maintenance at 3-month intervals during which the implant surface was treated with glycine, iodine, and laser bacterial reduction at each visit.12 After 3 months, there were no signs of inflammation, exudate, or bleeding while palpating the buccal or lingual aspect or while probing. After 1 year, the pocket depths had reduced to 4 mm to 5 mm and radiographic exam shows some possible bone remineralization (Figure 4).

MOVING FORWARD
New research and developments may alter the course of how implant complications are identified and treated. Patients who undergo implant therapy need to be closely followed. Oral health professionals must achieve the clinical skills—developed from careful, diligent training based on the latest research—necessary to maintain such patients. For example, Wilson et al13 found bacteria, encapsulated cement, and titanium fragments in the ulcerated soft tissue adjacent to dental implants. Considering these findings, clinicians must understand how to instrument around a dental implant to reduce the possibility of soft tissue contamination. Clinicians also need to consider that traditional periodontal flap procedures may still be necessary to treat peri-implantitis even after nonsurgical treatment with an endoscope.
Dental hygienists are well suited to use the dental endoscope in the treatment of peri-implantitis. Furthermore, the endoscope can be an effective preventive tool. Wilson14 used a dental endoscope to evaluate peri-implantitis in 42 test implants to inspect for residual cement. He found that residual cement was associated with adjacent peri-implantitis soft tissue lesions in 34 of these sites. If these implants were inspected with an endoscope at the time of restoration, more complete removal of residual cement would have been accomplished, with potentially fewer cases of peri-implantitis.
REFERENCES
- American Academy of Implant Dentistry. What Are Dental Implants? Available at: aaid-implant.o/g/dental-implants/what-are-dental-implants/. Accessed August 19, 2019.
- Wilson TG Jr. The positive relationship between excess cement and peri-implant disease: a prospective clinical endoscopic studyJ J Periodontol. 2009;80:1388–1392.
- Smeets R, Henningsen A, Jung O, Heiland M, Hammacher C, Stein JM. Definition, etiology, prevention and treatment of peri-implantitis—a review. Head Face Med. 2014;10:34.
- Carcuac O, Berglundh T. Composition of human peri-implantitis and periodontitis lesions. J Dent Res. 2014; 93:1083–1088.
- Farina R, Simonelli A, Minenna L, et al. Change in the gingival margin profile after the single flap approach in periodontal intraosseous defects. J Periodontol. 2015;86:1038–1046.
- Prathapachandran J, Suresh N. Management of peri-implantitis. Dent Res J (Isfahan). 2012;9:516–521.
- Gulati M, Govila V, Anand V, Anand B. Implant maintenance: a clinical update. Int Sch Res Notices. 2014;2014:908534.
- Schwarz F, Schmucker A, Becker J. Efficacy of alternative or adjunctive measures to conventional treatment of peri-implant mucositis and peri-implantitis: a systematic review and meta-analysis. Int J Implant Dent. 2015;1:22.
- Kuang Y, Hu B, Chen J, Feng G, Song J. Effects of periodontal endoscopy on the treatment of periodontitis; a systematic review and meta-analysis. J Am Dent Assoc. 2017;148:750–759.
- Slots J. Selection of antimicrobial agents in periodontal therapy. J Periodont Res. 2002;37:389–398.
- Abduijabbar T, Javed F, Kellesarian SV, Vohra F, Romanos GE. Effect of Nd:YAG laser-assisted non-surgical mechanical debridement on clinical and radiographic peri-implant inflammatory parameters in patients with peri-implant disease. J Photochem Photobiol B. 2017;168:16–19.
- Stein J, Hammacher C, Michael S. Combination of ultrasonic decontamination, soft tissue curettage and submucosal air polishing with povidone-iodine application for non-surgical therapy of peri-implantitis: 12 months clinical outcomes. J Periodontol. December 5, 2017. Epub ahead of print.
- Wilson TG Jr, Valderrama P, Burbano M, et al. Foreign bodies associated with peri-implantitis human biopsies. J Periodontol. 2015;86:9–15.
- Wilson T. The positive relationship between excess cement and peri-implant disease; a prospective clinical endoscopic study. J Periodontol. 2009;80:1388–1392.
From Dimensions of Dental Hygiene. September 2019;17(8):14,16–17.

