Sleep Disorder Detection in Children
Knowledgeable oral health professionals are uniquely positioned to identify patients early in the disease process.
This course was published in the February 2016 issue and expires February 28, 2019. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Define sleep breathing disorders.
- Discuss strategies for evaluating children for sleep breathing disorders.
- Identify treatment options for sleep breathing disorders.
Dental hygienists with knowledge of airway concepts have a unique opportunity to impact the lives of all patients, but particularly children. When approached from a medical model, the effects of a compromised airway are not evident until diagnoses like hypertension and cardiovascular disease begin to emerge. In contrast, oral health professionals can identify patients who are in the earliest stages of airway compromise when interventional measures can be implemented with the goal being prevention, rather than treatment. Although a physician must make the diagnosis of SDB, it can often be detected in the patient’s craniofacial development and occlusal dysfunction. Dentists and dental hygienists are uniquely positioned to identify these patients early in the disease process.
PATIENT EVALUATION
An airway screening should be conducted on every patient during the dental hygiene appointment. In this article, the discussion will be limited to children. The evaluation process begins when the patient is first greeted and continues throughout the appointment. At the first visit, a general assessment of fitness or body mass index can be made. An overweight child is more likely to have SDB due to excess fat stored around the neck and waist. OSA is more prevalent among boys who are overweight and have adenotonsillar hypertrophy.7 Observe the child’s posture. Frequently, children with SDB will have a forward head posture as this helps to open the airway, making it easier to breathe.8 Children with SDB may present with a long, adenoid face with venous pooling under the eyes, often known as allergy shiners. These features are signs that the child may be developing craniofacial changes related to airway issues.9 A sleep-deprived child may be wiggly, overactive, poorly behaved, and have difficulty following directions. Habitually snoring children are at high risk for social problems, poor academic performance, decreased attention, and anxiety/depression.10–12 Even children who snore occasionally demonstrate altered brain function and more delayed and effortful processing. These children also experience more behavioral problems than nonsnoring children.13 Except for periods of illness, mouth breathing and snoring should never be considered normal. Although children who snore may grow out of it as their body size increases to accommodate their tonsils and adenoids, the risk of cognitive impairment before this happens remains.
QUESTIONNAIRE
Administering a simplified airway questionnaire can be helpful in detecting SDB. A standard question is: Have you seen your child mouth breathe, snore, or stop breathing during the night? Snoring typically occurs when air passes between the tongue and soft palate, causing a vibration of the soft palate. A snoring sound may also be produced from the nose during inhalation. Children can produce the same loud snoring sound as adults, but typically their snoring is more of an effortful breathing, making diagnosis more challenging. Habitual snoring, defined as snoring three times per week or more, has been associated with hyperactive behavior in children as young as 3 and poor academic performance.14,15 Sleep fragmentation or disruption caused by snoring appears to play a role in causing dysfunctions. Benign snoring in adults may increase the risk of stroke, and it has been linked to neurological indications such as headaches and psychiatric disorders such as anxiety/mood disorders.13 Snoring independent of OSA may cause neurocognitive dysfunction and impaired daytime performance.11
The parent/caregiver should then be asked how loud the child’s snoring is. It appears that the greater the volume, the higher the risk for apnea. Finally, a formal sleep study should be conducted on any child who has a history of cessation of breathing during sleep.
A child’s movement during sleep is another important consideration. Sleep actigraphy is a measurement of movement occurring during periods of sleep. Children who move significantly throughout the night are not reaping the benefits of sleep. Many of the triggers for movement are airway related, warranting evaluation of sleep breathing patterns.
Asking if the child wakes feeling tired or groggy and/or is difficult to arouse is the next question. A positive response to this question could indicate poor quality or lack of sufficient sleep. A child with 10 hours to 12 hours of quality sleep should wake up refreshed and ready to go. Symptoms of fatigue, daytime sleepiness, and morning headaches are also indicative of SDB.
The parent/caregiver should then be asked if the child has difficulty completing assignments or has peer/conduct problems. Children with SDB often show signs of attention deficit hyperactivity disorder (ADHD).14 They may experience difficulty in school, behavioral problems, poor grades, and trouble completing homework.
The most important question is whether the child is a mouth breather. Up to 25% of children mouth breathe daily. Additionally, 78% of children who snore are daily mouth breathers.16 Closed-mouth breathing with the tongue in the roof of the mouth helps direct ideal maxillofacial growth. Nasal breathing reduces the collapsibility of the airway, promotes oropharygeal opening during sleep, warms and humidifies the air, has an antibacterial affect, and promotes better oxygen transfer in the lungs.17
HEALTH HISTORY REVIEW
Beyond the screening questionnaire, the medical and dental history may provide evidence of SDB symptoms. Attention deficit disorder or ADHD are commonly found in children with SDB.18 These children typically take stimulants, such as amphetamine or dextroamphetamine. Parents may report frequent ear infections, sore throats, and tonsillitis with high use of antibiotics. Children who mouth breathe often have pressure equalization tubes placed at a young age. Recurrent otitis media may cause speech or language delays because of temporary hearing loss. Poor or excessive weight gain should be suspect. Bruxism is frequently reported and can be used as a clinical marker for SDB.19 In fact, parents may have such concern over their child’s grinding habit that dental appointments may be scheduled for this reason alone. Bruxism is believed to be a reaction to protect the airway when respiratory effort begins. The theory is that it assists in preventing airway collapse by increasing the muscle tone in the oropharynx. Enuresis may be present because of fragmented sleep. The recurrent fragmentation may increase their arousal threshold beyond what a full bladder will stimulate.20 Thumb or finger sucking may be used as a technique by the child to reposition the tongue out of the airway and decrease inspiratory resistance.
EXAMINATION
Several areas of the mouth can provide indications of SDB. We refer to these as the five T’s: teeth, tissue, tongue, tonsils, and throat.
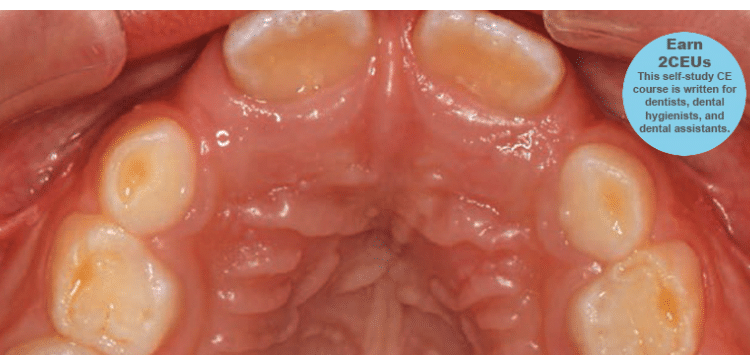
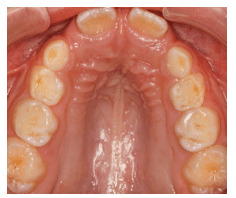
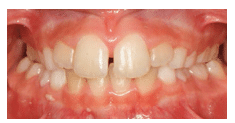
Teeth: Occlusal wear is often the most striking feature. This wear occurs during bruxism at night when the mouth is dry and there is more friction on the teeth. Evidence of mouth breathing (chronically dry chapped lips) is common. The prevalence of caries on the anterior teeth is increased in mouth-breathing children. Pediatric patients with UARS and OSA may have a high, narrow hard palate, a long face, and a Class II malocclusion (Figure 1). Retroclined or crowded dentitions and/or crossbites indicate abnormal arch growth possibly due to improper tongue placement and breathing patterns (Figure 2).
Tissue: Inflamed, red gingival tissue, especially in the anterior region, is additional evidence of mouth breathing. Periodontal diseases may be more common in patients with SDB due to abnormal growth in inflammatory cell proliferation and increased pro-inflammatory cytokines and other inflammatory mediators.
Tongue: The size, placement, and movement of the tongue should be evaluated. Macroglossia is a marker for SDB. The majority of children, however, will not have large tongues. Instead, the arch will be inadequately developed due to poor tongue placement, giving the illusion that the tongue is too large. The finest orthodontic tool for promoting maxillary positioning and arch form is the tongue. Poor tongue placement can be caused by adenotonsillar hypertrophy, ankyloglossia, or a combination of the two. Ankyloglossia can prevent the tongue from pressing into and shaping the maxilla, which then results in a high, narrow, hard palate. Tongue ties can be anterior and posterior. When the tongue is raised, the frenum pull creates a heart-like shape to the tip of the tongue. A lesser-known type of ankyloglossia is a posterior tongue tie. The attachment point is the same but the fibers do not travel to the tip of the tongue. Instead, as the tongue is raised, a depression is formed in the dorsum. When asked, patients cannot stick their tongues out far. Instead, the tips will become rounded and blunted, and they cannot maintain space between the ventral surface and the lower anterior incisal edges. They should be asked to try to open their mouths with the tongue held at the incisive foramen. Tongue ties requiring treatment limit the maximum opening by at least 30%.
Tonsils: These may be enlarged and frequent throat infections may be reported. An evaluation of the tonsils is done by pressing down on the posterior portion of the tongue with the mouth mirror and asking the child to say ah. Tonsils can vary in size, from not visible (Grade 1) to touching each other and completely blocking the throat (Grade 4). Grade 2 obstructs the airway by 25% and Grade 3 by 75% (Figure 3). The tonsils, however, are not the most important point of airway obstruction; it is the adenoids that cause more trouble. The problem is that adenoids cannot be visually inspected without an endoscopic procedure. Therefore, small tonsils should not discourage clinicians from further examination. As such, children suspected of SDB are asked to get a cephalometric radiograph or cone-beam computed tomography (Figure 4).
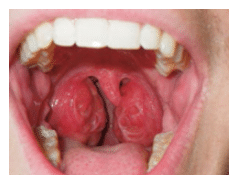
Throat: The airway evaluation can take place during the oral cancer examination. The Mallampati Classification was originally developed to predict the ease of endotracheal intubation. It is a visual inspection of the distance from the base of the tongue to the soft palate. The patient maximally protrudes his or her tongue and the anatomy of the oropharynx is evaluated. Class I exhibits a soft palate, uvula, fauces (arched opening in back of the mouth), and tonsillar pillars. In Class II, the fauces are visible, but in Class III the opening is not evident. Class IV is when the soft palate is not visible. Patients in Class III and Class IV may be more prone to anatomic airway obstruction due to the large size of the tongue or small size of the oral cavity (Figure 5).21 The tonsillar pillars and uvula may be red and edematous in patients who snore or have recurrent infections (Figure 6). We have found that children (and adults) who say something like “gnnn” as opposed to “ah” may be demonstrating an airway protective reflex that could be predictive of SDB. Several other protective behaviors are readily seen in the dental appointment. Pushing the tongue against the mouth mirror and dental instruments, gagging easily, and difficulty taking X-rays and impressions are common protective maneuvers. These patients may also be sensitive to tastes, smells, and textures.
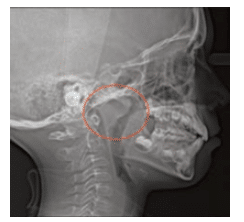 FIGURE 4. The adenoids are highlighted in this cephalometric radiograph.
FIGURE 4. The adenoids are highlighted in this cephalometric radiograph.
FIGURE 5. Only the hard palate is visible in Mallampati Class IV.
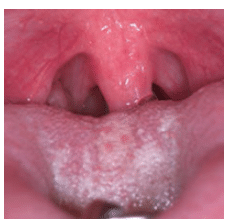
FIGURE 6. The red palatopharyngeal arch and edematous uvula are caused by snoring and narrow fauces.
DATA COLLECTION
Once SDB is suspected, objective data should be gathered. There are several tools that can be used to gain additional information. The gold standard is the polysomnogram (PSG) sleep study. This test provides a comprehensive look at sleep architecture. Any child who has experienced a witnessed apneic episode during sleep should have a PSG. There are several drawbacks to PSGs, however, including the need for the child to sleep in the lab in an unfamiliar environment. Additionally, the typical PSG analysis focuses on apnea and may disregard inspiratory flow limited breathing.
In addition to the sleep questionnaire, other tools can be used for screening pediatric patients. Parents/caregivers can record a video of their child sleeping. The recording should be representative of a typical night, demonstrating mouth breathing, snoring, apnea, or body movement. Cardiopulmonary coupling (CPC) is a less invasive tool that is approved for use on children as young as 6 months. The CPC is a single line electrocardiogram that monitors parasympathetic and sympathetic events during sleep, recording sleep quality. CPC is not a diagnostic tool, but rather confirms the presence of poor sleep. Restorative sleep is parasympathetic in nature. Sympathetic activity during sleep can be respiratory events or body movements, neither of which allows beneficial sleep. Children require four times as much parasympathetic as sympathetic sleep to remain healthy. Another tool is a high-resolution pulse oximeter that can be used particularly in older children. The pulse oximeter records pulse rate and oxygen saturation during sleep. Software analyzes the data gathered and provides the number of desaturations that qualify as apnea during the night. Because dentists are not able to diagnose medical conditions, patients should never be told they have apnea. Rather, oral health professionals should show patients the data indicating the possibility of sleep apnea and recommend they see their physician.
TREATMENT OPTIONS
The most common referrals for patients with sleep disorders are to otolaryngologists (ENT), orthodontists, and myofunctional therapists. When the adenoids and tonsils are the main point of obstruction, a pediatric ENT may be the only referral required. Removal of the tonsils and adenoids may permit proper use of the oral-facial and tongue muscles. The goal is to accomplish closed mouth breathing with the tongue in the roof of the mouth to help direct ideal growth. Once the child reaches 7, craniofacial and oronasal changes increase the risk of adenoid and tonsil removal not resolving the airway issue. In this case, palatal expansion by an orthodontist can give the tongue adequate room for a healthy airway and assistance in occlusal correction (Figure 7). A traditional orthodontic approach has been to wait for permanent tooth eruption before intervention. This will maintain an unhealthy airway for years, increasing the risk of poor outcomes.
Dental hygienists may also be involved by earning the orofacial myofunctional therapist (OMT) accreditation. This designation requires additional training in evaluating orofacial myofunctional disorders that may affect breastfeeding, craniofacial growth, chewing, swallowing, speech, and occlusion. OMTs teach patients proper tongue placement, breathing techniques, and healthy swallowing patterns, as well as directing the surgical referral of patients with ankyloglossia.
CONCLUSION
A healthy airway is required for both oral and overall health. Dental hygienists are uniquely qualified to detect symptoms of SDB among children. During routine assessment at recare visits, dental hygienists may observe evidence of SDB. As they are well versed in assessment, evaluation, and patient education, dental hygienists are the natural choice to discuss airway problems with parents/caregivers.
References
- Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000;1:21–32.
- Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:242–252.
- Chen W, Kushida CA. Nasal obstruction in sleep-disordered breathing. Otolaryngol Clin North Am. 2003;36:437–460.
- Gold AR, Dipalo F, Gold MS, Broderick J. Inspiratory airflow dynamics during sleep in women with fibromyalgia. Sleep. 2004;27:459–466.
- Woodson BT. Expiratory pharyngeal airway obstruction during sleep: a multiple element model. Laryngoscope. 2003;113:1450–1459.
- Gozal D, Hakim F, Kheirandish-Gozal L. Chemoreceptors, baroreceptors, and autonomic deregulation in children with obstructive sleep apnea. Respir Physiol Neurobiol. 2013;185:177–185.
- Li AM, Au CT, Ng SK, et al. Natural history and predictors for progression of mild childhood obstructive sleep apnoea. Thorax. 2010;65:27–31.
- Solow B, Skov S, Ovesen J, et al. Airway dimensions and head posture in obstructive sleep apnoea. Europ J Orthod. 1996;18:571–579.
- Zettergren-Wijk L, Forsberg CM, Linder-Aronson S. Changes in dentofacial morphology after adeno-/tonsillectomy in young children with obstructive sleep apnoea—a 5-year follow-up study. Eur J Orthod. 2006;28:319–326.
- Blunden S, Lushington K, Lorenzen B, et al. Neuropsychological and psychosocial function in children with a history of snoring or behavioral sleep problems. J Pediatr. 2005;146:780–786.
- O’Brien LM, Mervis CB, Holbrook CR, et al. Neurobehavioral implications of habitual snoring in children. Pediatrics. 2004;114:44–49.
- Urschitz MS, Eitner S, Guenther A, et al. Habitual snoring, intermittent hypoxia, and impaired behavior in primary school children. Pediatrics. 2004;114:1041–1048.
- Barnes ME, Huss EA, Garrod KN, et al. Impairments in attention in occasionally snoring children: an event-related potential study. Dev Neuropsychol. 2009;34:629–649.
- Gill AI, Schaughency E, Galland BC. Prevalence and factors associated with snoring in 3-year olds: early links with behavioral adjustment. Sleep Med. 2012;13:1191–1197.
- Kurnatowski P, Puty?ski L, Lapienis M, Kowalska B. Neurocognitive abilities in children with adenotonsillar hypertrophy. Int J Pediatr Otorhinolaryngol. 2006;70:419–424.
- Hultcrantz E, Löfstrand Tideström B. The development of sleep disordered breathing from 4 to 12 years and dental arch morphology. Int J Pediatric Otorhinolaryngol. 2009;73:1234–1241.
- Guilleminault C, Sullivan SS. Towards restoration of continuous nasal breathing as the ultimate treatment goal in pediatric obstructive sleep apnea. Enliven: Pediatr Neonatol Biol. 2014;1:1.
- Bonuck KA, Chervin RD, Cole TJ, et al. Prevalence and persistence of sleep disordered breathing symptoms in young children: a 6-year population-based cohort study. Sleep. 2011;34:875–884.
- Singh N, Chandwani B, Finkelmann M, et al. Sleep bruxism-related tooth wear as a clinical marker for pediatric sleep-disordered breathing. Paper presented at: 21st Annual American Academy of Dental Sleep Medicine Meeting; June 8, 2012; Boston.
- Cohen-Zrubavel V, Kushnir B, Kushnir J, Sadeh A. Sleep and sleepiness in children with nocturnal enuresis. Sleep. 2011;34:191–194.
- Liistro G, Rombaux P, Beige C, Aubert G, Rodenstein DO. High Mallampati score and nasal obstruction are associated risk factors for obstructive sleep apnoea. Eur Respir J. 2003;21:248–252.
From Dimensions of Dental Hygiene. February 2016;14(02):45–48.



