
Simple Strategies for Surface Disinfection
Clinicians need to be well informed about the properties of the products they use for cleaning and disinfecting surfaces.
As globalization, or the interconnectedness and interdependence of populations and countries around the world, increases, so does the risk of disease transmission.1 International travel, just one facet of globalization, has experienced significant growth. For example, in 1980, approximately 227 million individuals traveled internationally via airplanes. In comparison, more than 1 billion people crossed international borders via airplane travel in 2012.1 As more people and goods cross international borders, so do diseases such as hepatitis B virus (HBV), tuberculosis, severe acute respiratory syndrome, Middle East respiratory syndrome, and Avian influenza.2 As such, maintaining infection control protocols in all facets of health care is imperative to the safety of both patients and clinicians.
Adherence to proper infection control protocols can minimize or prevent disease transmission among patients and the dental team. By strictly following evidence-based methods of infection control as outlined by the United States Centers for Disease Control and Prevention (CDC), dental offices will remain safe clinical environments.3
According to the CDC,3 there are four principles of infection control:
- Take action to stay healthy
- Avoid contact with blood and other infectious body substances
- Ensure patient care items are safe for use
- Limit the spread of blood and other infectious body substances
A clear understanding of the CDC’s Guidelines for Infection Control in Dental Health-Care Settings—2003 is necessary to implement and achieve an effective infection prevention protocol.3 These recommendations are designed to help oral health professionals properly prepare the dental operatory for patient treatment. Heat sterilization is typically used on equipment and instruments that can withstand high heat.4 For those devices that are not heat-tolerant or cannot be removed for heat sterilization, chemical sterilization is used to supplement infection control efforts.4 Those surfaces with potential for exposure to blood or other potentially infectious materials must be disinfected with an intermediate-level disinfectant.3
ENVIRONMENTAL SURFACES
The CDC and Organization for Safety, Asepsis and Prevention (OSAP) categorize environmental surfaces as either clinical contact surfaces or housekeeping surfaces. Clinical contact surfaces are those directly touched by instruments, devices, hands, or gloves with direct contact with blood or other potentially infectious materials. These include but are not limited to light handles, switches, delivery trays, and chairside computers.
Housekeeping surfaces are not directly touched during the delivery of care and include walls, floors, sinks, and countertops. Unless they become visibly contaminated, housekeeping surfaces require only regular cleaning with soap and water to remove soil and dust.5
While environmental surfaces are not often associated with transmission of disease in the dental setting, some of the most harmful health care-associated pathogens can survive on these surfaces for days, weeks, or months. For example, HBV can remain infectious when contained in dried blood on environmental surfaces for at least 1 week and possibly longer.6,7 Reducing the potential for disease transmission via these surfaces is an important component of infection control protocol. Although it is impossible to achieve 100% sterilization on surfaces in the dental operatory, high levels of disinfection can be achieved through proper technique, use of barriers, and surface disinfectants.
Sterilization uses heat or chemicals to kill all microorganisms including spores. Disinfection, on the other hand, chemically destroys most microorganisms on items or objects but does not eliminate all bacterial spores. Using a combination of barriers and surface disinfection in the infection control routine can significantly reduce the risk of disease transmission.8
Surfaces that cannot be easily cleaned due to their inability to withstand moisture or chemical exposure or their size or shape should be covered with fluid-resistant barriers during delivery of care. These items may include switches, irregular surfaces, digital radiography sensors, and keyboards.3,4 These barriers must be replaced between patients with clinicians being careful to minimize the possibility of cross-contamination. If contamination inadvertently occurs, the surface must be cleaned and disinfected before another barrier is placed.
CLEAN AND DISINFECT
Cleaning should be conducted first to remove debris and visible contamination. It is a critical step to effective infection control because debris that is left behind can interfere with the efficacy of disinfection.9 Cleaning should be followed by disinfection, which eliminates, inactivates, or destroys most pathogens to reduce the risk of transmission.3,5
OSAP recommends that “areas should be cleaned and then saturated with enough disinfectant for the surfaces to remain moist for the required contact time without evaporation.”9 Two methods are acceptable to accomplish this goal. One is the spray (to clean)-wipe-spray (to disinfect) method. The second method uses premoistened wipes and suggests wipe (to clean)-discard-wipe (to disinfect). A clean wipe should be used for each surface. Use a sufficient number of wipes to ensure the surface remains damp for the appropriate clinical contact time.8,9
CHOOSING A SURFACE DISINFECTANT PRODUCT
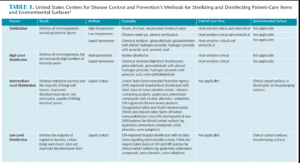
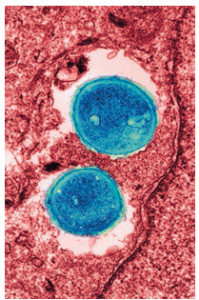
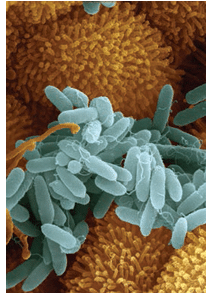
Surface disinfectants are categorized in three levels: low level, intermediate level, and high level (Table 1).3 High-level disinfectants are not typically used in dental offices, so they will not be discussed here. Product labels do not state the level of efficacy but rather make a label claim. Both low- and intermediate-level disinfectants claim “hospital disinfectant.” This means they are effective against three test organisms: Salmonella choleraesuis, Staphylococcus aureus (Figure 1) and Pseudomonas aeruginosa (Figure 2). Some low-level disinfectants may kill human immunodeficiency virus (HIV) and HBV, if stated on the label. They can be used as cleaners on surfaces that are not visibly contaminated with blood or other potentially infectious materials. These are most useful for cleaning housekeeping surfaces and clinical contact surfaces not otherwise contaminated.
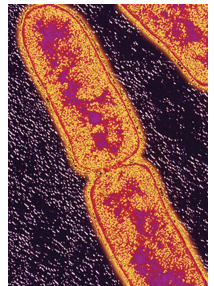
Intermediate-level disinfectants include the above claims but also are tuberculocidal. Potency against Mycobacterium tuberculosis (Figure 3) has been recognized as a substantial benchmark. The tuberculocidal claim, however, is used only as measurement of germicidal potency. Tuberculosis is not transmitted via environmental surfaces but rather through airborne routes. Because mycobacteria have some of the highest levels of resistance among vegetative bacteria, viruses, and fungi, any germicide with a tuberculocidal claim on the label is considered capable of inactivating a broad spectrum of pathogens, including less-resistant organisms such as bloodborne pathogens (eg, HBV, hepatitis C virus, and HIV). It is this broad-spectrum capability rather than the product’s specific potency against mycobacteria that is the basis of tuberculocidal chemicals for surface disinfection.10
Intermediate-level disinfectants can be used for both housekeeping surfaces and clinical contact surfaces that are soiled with blood or other potentially infectious materials. Most will have label claims stating bactericidal, fungicidal, virucidal, and tuberculocidal abilities. If the label claim says tuberculocidal, it is an intermediate-level disinfectant.5 Because it often is difficult to see blood or other potentially infectious material, an intermediate-level disinfectant is the agent of choice for most clinical contact surfaces.
The CDC and OSAP both recommend using personal protective equipment when cleaning and disinfecting, which includes heavy-duty utility gloves, face mask, protective eyewear, and protective gown. Chemicals can be absorbed or inhaled if personal protective equipment is not properly utilized.5
Before using a cleaner or disinfectant, the manufacturer instructions should be well understood. Contact times vary among products. Clinicians should search for products with reasonable contact times (less than 10 minutes). The label should be checked to determine if the product is ready to use or needs to be mixed.8
Understanding the chemical compounds or type of disinfectant used also is important. There are four standard types:
- Chlorine-based products contain chlorine dioxide and sodium hypochlorite and have a broad kill spectrum but limited shelf life. Sodium hypochlorite is no longer recommended, as it is not EPA registered.
- Phenolic solutions are water or alcohol based and are identified with prefixes and suffixes such as “phenol” or “phenyl.” They provide antifungal and antiviral properties.
- Iodophors are less irritating to the skin and identified with “iodi” or “iodo.”
- Quaternary compounds are not corrosive and have a lower kill spectrum.
All hospital-level disinfectants must contain an EPA registration number. The EPA requires manufacturers to test formulations by using accepted methods for microbial activity, stability, and toxicity to humans and animals.9
CONCLUSION
Each dental office has unique needs and there is no perfect disinfectant. Clinicians need to understand the CDC Guidelines and how they relate to daily practice. These are currently under revision and updates may be released in the future. By reading product labels and choosing appropriate products for the office’s needs, clinicians can be confident in their adherence to infection control protocols. Remember that stronger is not necessarily better, as stronger may mean more toxic to the user and the environment. The ideal surface disinfectant is broad spectrum; fast acting; active in the presence of organic matter; nontoxic; nonallergenic; nondamaging to surfaces such as metal, cloth, rubber or plastics; leaves no residual effect on treated surfaces; easy to use; and economical. While all products have some limitations, clinicians who are well versed in the active ingredients and strengths and weaknesses of the products they use will be prepared to enforce infection control protocol.11 Overall, the safety of staff, patients, and the environment must be considered when choosing a disinfectant. Clinicians who take the time to become knowledgeable about the properties of their chosen products are helping to ensure the efficiency of infection control efforts while minimizing the potential for disease transmission.
REFERENCES
- SUNY Levin Institute Globalization 101. Increased Global Travel. Available at: globalization101.org/increased-global-travel/. Accessed May 24, 2015.
- World Health Organization. Q&A Infectious Diseases. Available at: who.int/topics/infectious_diseases/qa/en. Accessed May 24, 2015.
- Kohn WG, Collins AS, Cleveland JL, et al. Guidelines for infection control in dental health-care settings—2003. MMWR Recomm Rep. 2003;52:1–61.
- Cuny EJ. Tips for effective surface disinfection. Dimensions of Dental Hygiene. 2014;12(6):26–30.
- CDC Guidelines: From Policy to Practice by OSAP. Annapolis, Maryland: Organization for Safety, Asepsis and Prevention; 2004.
- Laheij AMGA, Kistler JO, Belibasakis GN, Valimaa H, de Soet JJ, European Oral Microbiology Workshop 2011. Healthcare-associated viral and bacterial infections in dentistry. J Oral Microbiol. 2012;4:10.
- Bond WW, Favero MS, Petersen NJ, Gravelle CR, Ebert JW, Maynard JE. Survival of hepatitis B virus after drying and storage for one week. Lancet. 1981;1:550–551.
- Garland K. The surface disinfectant challenge. Access. 2010;24(1):19.
- Inside Dental Assisting. Q&A: All About Surface Disinfectants in the Dental Office. Inside Dental Assisting. Available at: dentalaegis.com/ida/2013/06/q-a-all-about-surface-disinfectants-in-the-dental-office. Accessed May 24, 2015.
- Molinari, JA, Jarte JA. Cottone’s Practical Infection Control in Dentistry. 3rd ed. Baltimore: Lippincott, Williams & Wilkins; 2010.
- Organization for Safety, Asepsis and Prevention. OSAP Provides Clinical Contact Surface Disinfectant Resources—2015. Available at: osap.org/?page=Disinf_Info. Accessed May 24, 2015.
From Dimensions of Dental Hygiene. June 2015;13(6):42,44,46–47.

