Safety First
Dental hygienists must remain up to date on topical anesthetic usage in order to reduce any possible risks.
Dental hygienists have become expert in managing patient discomfort and pain during dental hygiene procedures. Topical anesthetics play a valuable role in achieving comfort during therapies like scaling and root planing. Despite their extensive use and well-deserved reputation for safety, there are legal limitations to the use of certain topical preparations, and not all topicals are equal when it comes to safety. It is ultimately the responsibility of each dental hygienist to keep current regarding the safe use of these products, their appropriate integration into patient care, and how to respond with the best pain management strategy for each individual patient and situation.
USING TOPICAL AGENTS
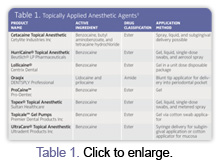
Topical anesthetics are commonly used for a variety of purposes in dentistry, such as minimizing the gag reflex, providing pain management at injection sites, and for localized soft tissue procedures. Available in a variety of commercial forms, topical anesthetics are dispensed as liquids, gels, creams, ointments, metered and unmetered sprays, subgingival gels, and eutectic mixtures (specific anesthetic agents that are more effective in combination and can provide greater depths of effective anesthesia compared to the individual agents acting alone). Application methods include the use of cotton swabs, sprays, patches, and subgingival delivery with blunt-tip syringes. For examples of available topical anesthetic products, see Table 1.
Compounded topical anesthetic agents are alternatives to standard commercially prepared agents; they are formulated by a compounding pharmacist. These preparations have similar effects compared to commercial products, although they can provide greater depths and durations of anesthesia.
When obtaining compounded products, it is important to be familiar with the pharmacy compounding law, which is part of the Food and Drug Administration (FDA) Modernization Act of 1997. A key provision of the compounding law states, “The compounded product must be individually prescribed for an identified patient.” This means that an office may not acquire a multidose package and then use it for multiple patients.1 A copy of the act and further information are available at www.fda.gov.
THE DANGERS OF OVERUSE
The FDA has also issued public health advisories to remind health care professionals that the overuse of benzocaine anesthetic sprays, such as those used in the dental office, can cause methemoglobinemia. The FDA is reviewing the safety data for these products to determine if additional action is needed.
Benzocaine sprays are used to anesthetize the mucous membranes of the mouth and throat when preparing patients for minor surgery, endoscopic procedures, and endotracheal intubation. This practice is not unlike the use of benzocaine sprays to manage gagging during radiographic procedures and taking impressions for dentistry. Methemoglobinemia, a disorder characterized by the presence of a higher than normal level of methemoglobin in the blood, is a known side effect of benzocaine sprays, but the risk is increased when practitioners use multiple sprays or sprays of longer duration than recommended for susceptible patients.2
Maximum dose recommendations for most topical products do not exist.3 The FDA has noted that some product instructions are confusing and may lead to product misuse.4 For example, one manufacturer’s package has two separate “Instructions for Use.” One insert makes no reference to dose, while the other indicates a “half-second spray” from an unmetered delivery device as the correct dose, which is extremely difficult to monitor or reproduce. To reduce the risk of adverse reactions and to avoid confusion, read the manufacturer’s instructions carefully, watch for updates, and ask manufacturers for clarification.
Questions arise when product literature recommends reducing the dose in “debilitated, medically compromised patients and young children.” How are appropriate doses determined? Are dose recommendations realistic to apply? These questions pose specific risk-benefit concerns when using topicals without established maximum safe limits, as well as for those with established maximum safe limits (such as tetracaine 20 mg) but that do not provide a reliable method of metering the delivered dose.
COMMONLY USED TOPICAL AGENTS

Benzocaine, the most commonly used topical anesthetic agent, is an ester that is minimally absorbed into the systemic circulation and, therefore, has a very low potential for toxicity in healthy individuals.5,6 It is available in liquid, gel, and spray forms in concentrations ranging from 6% to 20%. The most commonly used concentration in dentistry is 20%, which provides adequate topical effect in 30 seconds but requires a full 2 minutes for greatest effect. Duration is 5 to 15 minutes.
Dyclonine hydrochloride, formerly available as Dyclone® (0.5% solution), a nonester, nonamide, ketone topical anesthetic, provides a relatively safe and durable alternative to other topical agents.5 It has a slow onset (between 2-10 minutes) and a duration of about 30 minutes. It is particularly useful with a patient who has or may be susceptible to hypersensitivity to amides and esters. Currently, it is available through compounding pharmacies by prescription. Dyclonine hydrochloride is also an active ingredient in over-the-counter products such as Sucrets® Children’s, Regular Strength, and Maximum Strength lozenges and Cepacol® Sore Throat Sprays.7
Lidocaine, an amide, is an excellent alternative to esters and is especially useful when ester use is contraindicated such as when patients are allergic to esters or are unable to safely metabolize them.5 For use in dentistry, lidocaine is available in 2% to 5% concentrations in ointment, liquid, and gel forms. Most lidocaine topical products are available in ointment form and are packaged in multidose jars. Gels are packaged in 2% concentrations, while solutions are available in 2% and 4% concentrations. Onset is 2-10 minutes and duration is approximately 15 minutes.
In dentistry, many topicals are used in combination with each other. Combinations of drugs are not new. Eutectic mixtures of local anesthetics have provided enhanced topical benefits for years due to their enhanced ability to pass through skin and mucous membranes more quickly than individual drugs acting alone.8 An example of a eutectic mixture in dentistry is the subgingival local anesthetic gel, Oraqix®*, a combination of 2.5% lidocaine and 2.5% prilocaine. The onset of Oraqix is 30 seconds and the duration is approximately 20 minutes.
Tetracaine is another useful anesthetic drug. It is found only in topical form in dental applications and typically in combination with other topical anesthetics drugs. For example, Cetacaine®** is formulated to provide long-lasting topical anesthesia. It combines 14% benzocaine, a fast-onset, short-acting topical with 2% butamben, an intermediate-onset, intermediate-acting drug, and 2% tetracaine, a slow-onset and long-acting topical to provide Cetacaine’s potentially broader therapeutic range of topical effect.
PRECAUTIONS AND CONTRAINDICATIONS
Most topical anesthetic agents are formulated in higher concentrations than injectable anesthetic agents in order to be effective when applied directly onto skin or mucosal barriers. Although toxic reactions are quite rare, the relatively high concentrations of anesthetic drugs used in topicals carry a potentially greater risk of toxicity for susceptible individuals. Clearly, more overdose reactions are experienced with injectable local anesthetic drugs than with topical agents. Nonetheless, dental hygienists must consider this risk when applying topicals. They need to read all package inserts, monitor the volumes administered, and calculate the interval of time between successive applications. Adverse reactions are due to excessive or rapid absorption, idiosyncrasy, hypersensitivity, or decreased patient tolerance.6
Common, nonlife-threatening, local reactions include tissue sloughing, delayed hypersensitivity, redness, pain, and/or burning at the application site. The likelihood of local reactions is related to tissue trauma during application, prolonged contact with mucosa, and undiagnosed hypersensitivity. These reactions respond well to palliative treatment and/or future discontinuation of the agent. Benzocaine and tetracaine are more likely to cause contact sensitivity than other anesthetic drugs placed topically.6
Allergic reactions to ester topical agents, although rarely seen, are physiologically possible. Allergies to amide drugs are virtually unknown.3 Despite the rare allergenic potential of amide topical anesthetics, many multidose preparations of topicals do contain methylparaben, a preservative that was banned by the FDA in dental cartridges in 1984 due to an increased risk of hypersensitivity. In multidose topical preparations, methylparaben provides important antioxidant, bacteriostatic, and fungistatic properties while, unfortunately, increasing the risk of allergic response.9

Systemic reactions can occur with the use of both injectable and topical drugs. The use of excessive volumes increases the risk significantly. Other systemic reactions to topical anesthetic agents can range from subclinical to life threatening. They may manifest as mild symptoms of central nervous system (CNS) depression such as restlessness, agitation, and increased heart rate to signs and symptoms of more severe CNS depression, such as unconsciousness and convulsions. They may also manifest as mild to severe complications of methemoglobinemia including lethargy, cyanosis, and respiratory difficulty.9
Nonspray benzocaine topicals have a long, well-deserved record of safe use in dentistry and should continue to provide safe and useful anesthesia into the foreseeable future. However, in excessive volumes in spray form, benzocaine has been linked to a serious potential risk of methemoglobinemia.10 In addition to benzocaine, prilocaine also has a recognized potential of inducing methemoglobinemia in both topical and injectable forms.8,11
Certain underlying conditions may predispose an individual to the development of methemoglobinemia if benzocaine or prilocaine is administered. The FDA Advisory states that, “…patients with underlying breathing problems, such as asthma or emphysema, patients with heart disease, and those who smoke may be more susceptible …”12
Other medications, dyes, and additives are risk factors as well. While the potential for adverse reactions certainly exists, the majority of the incidents that led to this advisory did not occur due to the use of benzocaine in dentistry. However, it is critical that dental professionals evaluate the products they use in order to avoid the overuse of all anesthetics. Evaluating the use of any compounded topical anesthetic agents to assure that they are dispensed according to the law is also key to patient safety.
Unfortunately, there are no clear cut answers to all of these questions, especially where general recommendations fail to apply to specific situations. As questions arise regarding the safest use of topical products, dental hygienists should consider the risks and benefits for each patient (keep in mind that more is not always better), apply all topical anesthetic products in a judicious manner, be responsible for consulting all product and safety information that is available, talk with product representatives and local anesthesia experts, and seek out published product data from research.
REFERENCES
- FDA Modernization Act of 1997. Available at: www.fda.gov/fdac/features/2000/400_compound.html. Accessed September 26, 2007.
- Institute for Safe Medical Practices. Benzocaine-containing topical sprays and methemoglobinemia. Available at: www.ismp.org/newsletters/acutecare/articles/20021003.asp. Accessed September 26, 2007.
- Yagiela JA. Safely easing the pain for your patients. Dimensions of Dental Hygiene. 2005;3(5):20–22.
- Institute for Safe Medical Practices. Benzocaine spray products may cause life-threatening condition. Available at: www.ismp.org/pressroom/newsreleases.asp. Accessed September 26, 2007.
- Jastak JT, Yagiela JA, Donaldson D. Local Anesthesia of the Oral Cavity. Philadelphia: WB Saunders; 1995:110-116.
- The Pharmacogenetics and Pharmacogenomics Knowledge Base. Available at: www.pharmgkb.org. Accessed September 26, 2007.
- ADA/PDR Guide to Dental Therapeutics. 4th ed. Montvale, NJ: Thompson PDR; 2006:1:21.
- Tetzlaff JE. Clinical Pharmacology of Local Anesthetics. Boston: Butterman Heinemann; 2000:105.
- Malamed SF. Handbook of Local Anesthesia. 5th ed. St Louis: Elsevier Mosby; 2004:155-156.
- Abu-Laban RB, Zed PJ, Purssell RA, Evans KG. Severe methemoglobinemia from topical anesthetic spray: case report, discussion and qualitative systematic review. Canadian Journal of Emergency Medicine. 2003;3(1):51-56.
- Knobeloch L, Goldring J, LeMay W, Anderson H. Prilocaine-induced methemoglobiemia— Wisconsin, 1993. MMWR Morb Mortal Wkly Rep. 1994;43:655-657.
- Advisory on Benzocaine Sprays and Methemoglobinemia. FDA Patient Safety News. Available at: www.accessdata.fda.gov/scripts/cdrh/cfdocs/psn/printer.cfm?id=418. Accessed September 26, 2007.
From Dimensions of Dental Hygiene. October 2007;5(10): 20-22, 25.