 SELVANEGRA/ISTOCK/GETTY IMAGES PLUS
SELVANEGRA/ISTOCK/GETTY IMAGES PLUS
The Role of Vaccines in Combatting Covid-19
The development and implementation of COVID-19 vaccines are key to winning the battle against SARS-CoV-2 and its variants.
This course was published in the December 2021 issue and expires December 2024. The author has no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Identify the basic principles inherent in vaccine development and vaccination programs.
- Discuss SARS-CoV-2 infection and its variants.
- Note the challenges to the success of immunization efforts.
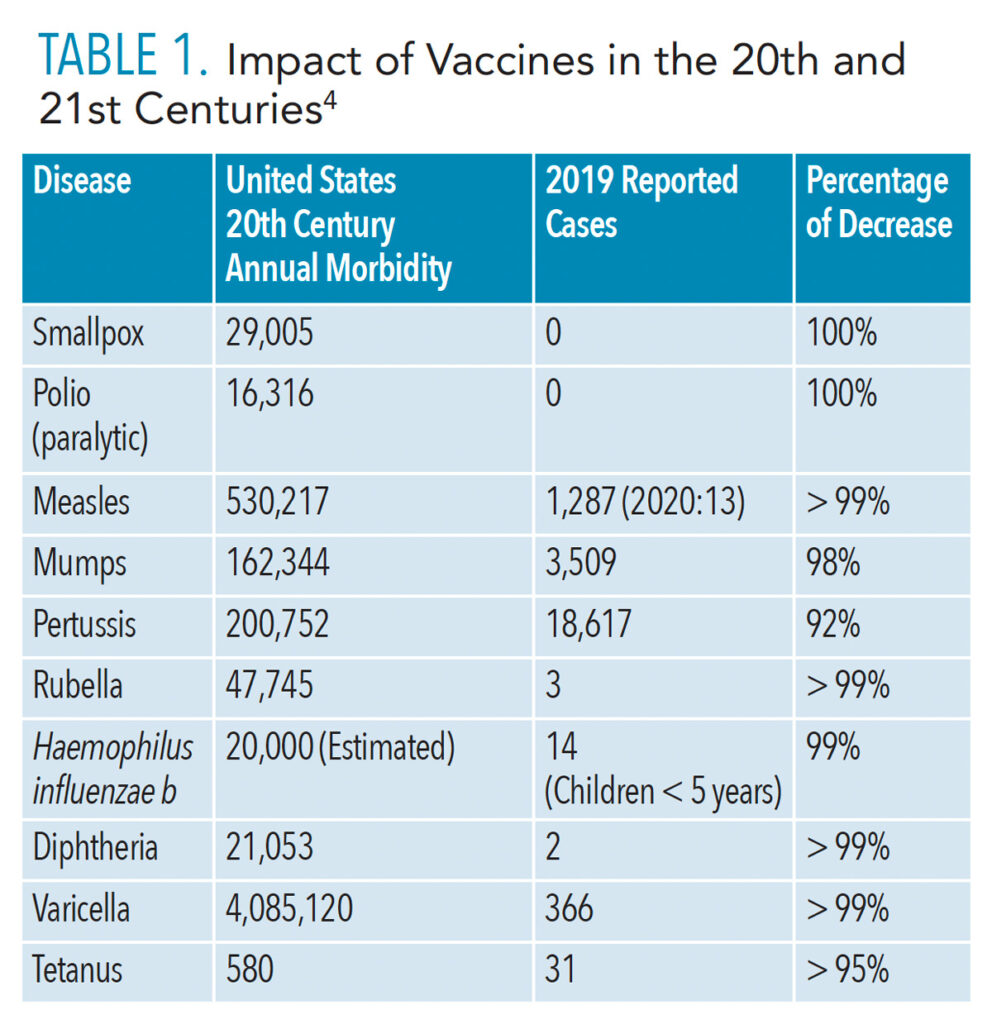
The ability of certain vaccines to control and greatly limit the spread of many infectious diseases—such as polio, diphtheria, measles, tetanus, and hepatitis B—is one of the most significant public health achievements of the 20th century.1 Vaccinations have changed the face of many infectious diseases by dramatically lowering their incidence in the population. Table 1 details this success against many previously common diseases. Routine childhood immunization has become an integral component of pediatric preventive medicine. In fact, most young adults have neither contracted nor seen previously common infections such as polio, diphtheria, tetanus, measles, mumps, and rubella, thanks to the long-standing success of childhood vaccination programs.
The historical success of public health vaccination programs is unprecedented. For example, the polio epidemic profoundly impacted vaccine development and the acceptance of universal vaccination by the American public.2 Yet, while the incidence of polio and other infectious diseases has been significantly reduced by immunizations, these diseases have not been eliminated. Adults and adolescents who escaped infection but are not immunized are at increased risk for a number of these diseases. The development and global implementation of COVID-19 vaccines represent the latest opportunity for widespread immunization as a critical strategy against the SARS-CoV-2 virus and its resultant variants.3,4
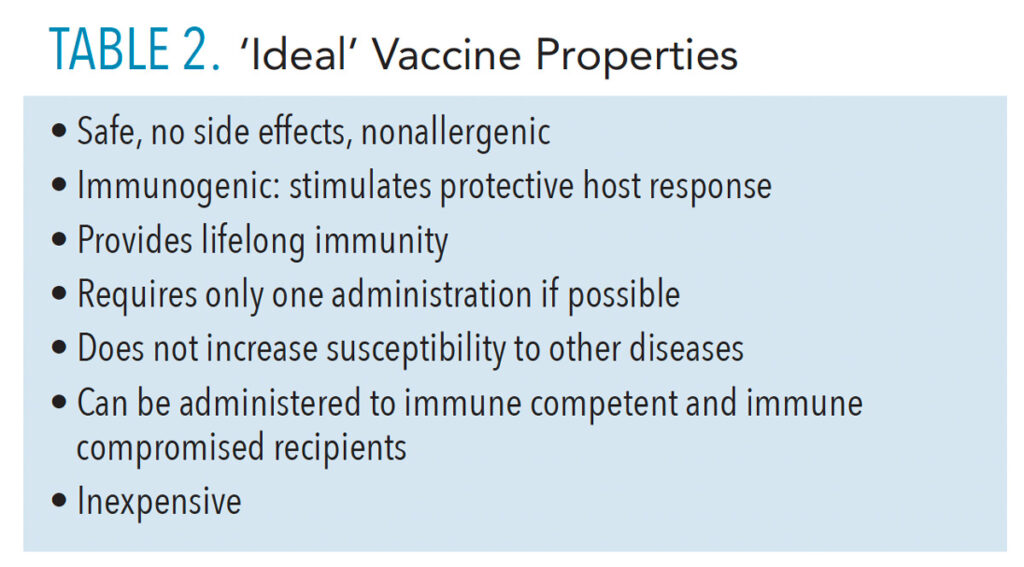
Vaccines that are effective and approved for clinical use must meet basic requirements throughout preclinical and clinical testing phases (Table 2).5 First and foremost, vaccine safety is a critical factor. In addition to established safety considerations during developmental phases, vaccines are consistently monitored and evaluated for possible adverse effects long after clinical trials are complete and they have been approved for use in the population.6 These after-approval evaluations are required for assuring vaccine safety. Healthcare professionals should be aware of these procedures used by the United States Centers for Disease Control and Prevention (CDC), US Advisory Committee on Immunization Practices, and US Food and Drug Administration (FDA), and other agencies in order to provide patients with helpful, up-to-date information.
Quality Control and Monitoring of Vaccine Safety
Vaccines approved by the FDA must meet high standards during laboratory investigation and human clinical trials. When manufacturers are able to obtain favorable results, the FDA then evaluates submitted data and can approve public release of the vaccine. Even after a vaccine is approved and released for use within the population, however, a strong multicheck system remains in place for ongoing monitoring of the vaccine. This involves a determined effort by multiple federal and state agencies along with local state health departments. They collect ongoing data concerning vaccine safety. One of the major monitoring systems is the Vaccine Adverse Event Reporting System, to which the above groups and the public can submit reports of possible side effects for further investigation.7
![TABLE 1. Impact of Vaccines in the 20th and 21st Centuries]() Potential Adverse Effects and Protection Safeguards
Potential Adverse Effects and Protection Safeguards
As successful as vaccines have been in providing protection against many pediatric and adult diseases, no vaccine is perfect, and post-immunization adverse events can occur. The overwhelming majority of these adverse reactions are minor and transient, lasting a few hours to a couple of days. These include injection site soreness, temporary myalgia, fatigue, and fever.8 Unfortunately, rare, serious reactions can also happen and may only be documented when large numbers of people receive the vaccine after FDA approval. These may be seen in one individual out of hundreds of thousands to millions of recipients. In some instances, the event may be so rare that it cannot be appropriately investigated. Ultimately, continued use of vaccines within the population requires that the benefits of immunization against disease far outweigh potential risks.
Attaining Successful Herd Immunity
Herd immunity occurs when a high percentage of the population becomes immune to a specific disease. It is much more difficult for the causative virus or bacteria to spread when enough people are immune and resistant to infection. As a result, the whole community becomes protected—not just those who are immune. That is an important prerequisite for attaining successful herd immunity.
An individual can become immune either by responding to vaccination (ie, artificial active immunity) or in the case of some diseases, recovering from the disease itself (ie, natural active immunity). One major reason for using mass vaccination is to rapidly increase immunity in a population during an existing or potential infectious disease outbreak, thereby limiting the morbidity and mortality that might result.9
In addition to protecting themselves, immune individuals are also able to protect other still vulnerable people in the population. These include those who are unable to receive the vaccine, those who fail to develop protective immune responses after vaccination, those who choose not to be vaccinated, and young children who may not be eligible for vaccination
A large “immune herd” provides protection to these groups and the other small numbers of susceptible people because the disease has little opportunity to spread within the community. The number and percentage of immune individuals needed to achieve herd immunity depends on the contagiousness of the disease. Measles, for example, is among the most infectious respiratory pathogens and requires a high percentage of immune people (95%) to reach herd immunity.10 While SARS-CoV-2 virus is less contagious, it is still more transmissible than other common respiratory pathogens such as influenza viruses and rhinoviruses. The emergence of more infectious viral variants during the COVID-19 pandemic has further complicated estimates for achieving herd immunity. Original recommended levels for herd immunity required approximately 70% of the population be immune, preferably through vaccination. The much more infectious Delta variant, which has surged through the country, has caused that target figure to rise to 80% to 90%.11
 A common question asked about herd immunity and SARS-CoV-2 viral disease is whether those who contract COVID-19 and recover are then protected from re-infection. While people may be able to develop some protection, there are major problems with relying on widespread community infection to create herd immunity against COVID-19.
A common question asked about herd immunity and SARS-CoV-2 viral disease is whether those who contract COVID-19 and recover are then protected from re-infection. While people may be able to develop some protection, there are major problems with relying on widespread community infection to create herd immunity against COVID-19.
It’s not clear how long an individual is protected from SARS-CoV-2 re-infection after recovering from COVID-19. Scientists are continuing to investigate how long natural active immunity and immunological memory can protect. This has become more important with the emergence and spread of increasingly contagious viral variants during the course of the pandemic. Ongoing clinical studies also are examining how much of the antibodies produced following natural infection with earlier variants can cross-react with corresponding antigens from more recent, infectious variants, such as Delta.
Accumulated data since early on in the pandemic show that a significant percentage of those who contracted and recovered from COVID-19 continue to experience prolonged health issues.12 As many as one in three individuals who recover have multiple symptoms for weeks to months later, including fatigue, memory problems, and inability to focus. Also post-COVID-19, damage to lungs, heart, kidney, liver, and other organs is being detected in all age groups.12 Currently, more than 4.5 million Americans have contracted COVID-19.13 Hospitals and other healthcare facilities have come close to being overwhelmed when caring for those who are severely ill. Millions more remain susceptible to SARS-CoV-2 infection. The potential impact for damage to the healthcare system could be substantial if/when susceptible individuals are infected, develop long-term pathologies, or need to be treated in a hospital setting.
Achieving herd immunity against COVID-19 has also become difficult because of widely circulating virus variants and persistent hesitancy within the population to receive vaccines. Even with vaccination rates increasing, SARS-CoV-2 variants and coronaviruses will remain a global threat. However, by vaccinating as large a share of the population as possible, this virus may ultimately move from a pandemic to the much more manageable endemic category of infectious diseases.
New Microbial Strains/Variants and Vaccine Success
Viruses are always mutating. A new strain or variant can possess one or more modifications that differentiate it from others already in circulation. Multiple SARS-CoV-2 variants have been documented throughout the world during the current pandemic. During infection by certain viruses, such as influenza and SARS-CoV-2, replication of their nucleic acid can undergo “antigenic drift.” This leads to copying errors, which cause mutations within the viral genetic material. Subsequently, alterations may occur in surface proteins or other antigens.
Rates of mutation vary among different viruses; influenza viruses mutate faster than coronaviruses. Antigenic drift is a major reason why new flu vaccines typically need to be developed for each upcoming flu season. Also, RNA viruses mutate faster than DNA viruses, and single-stranded viruses mutate faster than double-strand viruses. Basically, the more a virus can replicate, the greater the opportunity for chance mutations. These genetic changes may cause the virus to cause more serious disease or milder infections. In addition, while some mutations may not induce meaningful changes in viral properties, others can give the virus new characteristics, including increased virulence and ability to escape host immune defenses. In the case of SARS-CoV-2, mutations to the spike protein may impact the ability of the virus to attach onto host cell angiotensin-converting enzyme 2 receptors (which supply the entry point for coronaviruses) and to bind with host antibodies.10
Multiple SARS-CoV-2 variants have emerged during this pandemic. An interagency group established within the US government was organized early on in the pandemic. This SARS-CoV-2 Interagency Group meets regularly and is focused on the rapid characterization of emerging variants and actively monitors their potential impact on critical SARS-CoV-2 countermeasures, including vaccines, therapeutics, and diagnostics.
There are four classifications of SARS-CoV-2 variants: variants being monitored, variants of interest, variants of concern (VOC), and variants of high consequence. Of these, VOC are under the closest surveillance.14 Potential consequences caused by the appearance of VOC in a susceptible population include: more rapid transmission compared to viruses without the new mutation; milder or more severe disease; the ability to evade detection by specific serological tests; decreased susceptibility to therapeutic agents (eg, monoclonal antibodies and antivirals); and ability to evade natural or vaccine-induced immunity.
Table 3 (page 31) lists the current COVID-19 VOCs, along with representative properties for the four most common in the US. At the present time, the Delta variant is accounting for more than 99% of all new COVID-19 cases.15 It is far more transmissible than the original virus, replicates much faster than both the original pandemic virus and earlier variants, and can cause more severe disease, especially among unvaccinated individuals.16 As of this writing, the recently detected Lambda and Mu variants are not included on the US VOC list.16,17
Breakthrough Infections
A primary goal for manufacturers and the FDA’s vaccine approval process is to maximize a vaccine’s success, while minimizing the risk for adverse events and keeping potential post-vaccination infections to as low a level as possible. In addition to occasional side effects following immunization, breakthrough infection after vaccination is possible. Vaccines, however, are not 100% effective at preventing infection, and thus, some who are fully vaccinated can still get COVID-19. Clinical studies of vaccinated and unvaccinated individuals in a number of countries continue to show the risk of SARS-CoV-2 infection, hospitalization, and death from COVID-19 is much lower in vaccinated people than unvaccinated individuals, and yet, some fully vaccinated people will contract viral infection, and fewer may be hospitalized or even die from COVID-19.18,19
The CDC defines a COVID-19 vaccine breakthrough infection as “the detection of SARS-CoV-2 RNA or antigen in a respiratory specimen collected from individuals greater than or equal to 14 days after they have completed all recommended doses of an FDA-authorized COVID-19 vaccine.”20 To keep track of these cases, the CDC continues to collect data on hospitalized or fatal COVID-19 vaccine breakthrough cases. As of September 13, 2021, there were 15,790 reported cases.20
The CDC, World Health Organization, and other global institutions continue to investigate post-immunization breakthrough infections. These may happen due to impaired immunity, a weak response to the vaccine with little immunity memory, presence of SARS-CoV-2 infection at the time of vaccination, or evasion of immune response by emerging strains/variants. An individual may fail to immunologically respond to the vaccine by not producing protective antibodies and T-cells against SARS-CoV-2. Remember, even though a vaccine may have a 95% efficacy in clinical trials, that still means one in 20 will not respond to the vaccination regimen. The percentage of the population that does not develop a protective immune response may also increase if recipients are immune compromised or have other serious comorbidities.
![TABLE 3. Current SARS-Co V -2 Variants of Concern in the United States]() COVID-19 Vaccine Boosters
COVID-19 Vaccine Boosters
There was little doubt among infectious disease specialists and clinicians as to the necessity for COVID-19 booster injections after the initial immunization regimen. Discussion concerning booster doses has intensified, especially since the Delta variant surged among unvaccinated individuals and documentation of a low but increasing incidence of breakthrough infections in fully vaccinated individuals. While the FDA recently approved boosters for immune compromised persons and those age 18 and older, there is still disagreement among some infectious disease specialists and scientists concerning the timing for boosters. However, even as this issue is being studied and resolved, available vaccines remain very effective in combating SARS-CoV-2 illness, hospitalization, and death, even against the Delta variant.
There are myriad reasons why COVID-19 boosters are needed. First, SARS-CoV-2 is an RNA virus, and RNA viruses are known to mutate over time. Mutations that develop in susceptible hosts have already led to the rise of variants. SARS-CoV-2 also has a high rate of mutation. Global data have suggested that the virus has undergone more than 12,000 mutations since the start of the pandemic.21 Each major COVID-19 wave has been caused by a variant that possesses certain properties, which allow it to escape from host immune defenses. Furthermore, SARS-CoV-2 is not seasonal flu. It will not disappear during certain seasons like influenza, and it can cause far more damage during and after infection than influenza. To protect against future variants, the incidence of viral infection must be slowed.
Whether boosters will continue to be needed every 6 months or 8 months remains unknown. It all depends on how long the active vaccine-induced immune responses last and how well the body’s immunological memory capabilities can offer protection from current and emerging viral mutations.
Conclusion
Two years into the SARS-CoV-2 pandemic and much about it remains a mystery. Keeping up to date on the evidence-based research regarding COVID-19 and its vaccines is key to providing solid information to patients as well as supporting informed personal health decisions.
Author’s Note
This article provides a brief coverage of topics. The reader is urged to refer to more detailed vaccine publications for additional information,3,4 as well as consult science-based and clinical publications about SARS-CoV-2 infection and COVID-19 disease.
References
- United States Centers for Disease Control and Prevention. Ten great public health achievements—United States 1900-1999. Morbid Mort Wkly Rpt. 1999;48:241–243.
- Paul JR. A History of Poliomyelitis. New Haven, Connecticut: Yale University Press; 1971.
- Plotkin S, Orenstein W, Offit P. Vaccines. 6th ed. Philadelphia: Saunders; 2012.
- United States Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases. Available at: cdc.gov/vaccines/pubs/pinkbook/ appendix/index.html. Accessed November 15, 2021.
- Molinari JA, Terezhalmy GT. Immunizations for dental healthcare personnel. In: Molinari JA, Harte JA, eds. Cottone’s Practical Infection Control in Dentistry. 3rd ed. Baltimore: Lippincott Williams and Wilkins; 2010:89–100.
- The History of Vaccines. Vaccine development, testing, and regulation. Available at: historyofvaccines.org. Accessed November 15, 2021.
- Molinari JA. Quality control and monitoring of vaccines. Inside Dent. 2020:53–58.
- Chapin-Bardales J, Gee J, Myers T. Reactogenicity following receipt of mRNA-based COVID-19 vaccines. JAMA Insights. 2021;225:2201–2202.
- Heyman DL, Aylward RB. Mass vaccination: when and why. Mass Vaccinations: Global Aspects—Progress and Obstacles. Plotkin S, ed. Berlin: Springer-Verlag; 2006:1–16.
- Davaro RE, Fraire AE, Woa BA, Welsh RM, Kradin R. Measles virus. Viruses and the Lung. 2013;71–78.
- University of Missouri. COVID-19 Vaccine Key to Reaching ‘Herd Immunity’ Available at: muhealth.org/our-stories/covid-19-vaccine-key-reaching-herd-immunity#. Accessed November 15, 2021.
- Chippa V, Aleem A. Post acute coronavirus (COVID-19) syndrome. Available at: statpearls.com/pharmacist/ce/activity/49975. Accessed November 15, 2021.
- US Centers for Disease Control and Prevention. COVID-19 Data Tracker. Available at: cdc.gov/coronavirus/2019-ncov/covid-data/covidview/index.html. Accessed November 15, 2021.
- Boehm E, Kronig I, Neher RA, et al. Novel SARS-CoV-2 variants: the pandemics with the pandemic. Clin Micobiol Infect. 2021;27:1109–1117.
- US Centers for Disease Control and Prevention. Monitoring variant proportions. Available at: covid.cdc.gov/covid-data-tracker/#variant-proportions. Accessed November 15, 2021.
- World Health Organization. Tracking SARS-CoV-2 Variants. Available at; who.int/en/activities/tracking-SARS-CoV-2-variants/. Accessed November 15, 2021.
- US Centers for Disease Control and Prevention. SARS-CoV-2 Variant Classifications and Definitions. Available at: cdc.gov/coronavirus/2019-ncov/variants/variant-info.html. Accessed November 15, 2021.
- Bernal JL, Andrews N, Gower CD, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Eng J Med. 2021; 385:585–594.
- Griffin JB, Haddix M, Danza P, et al. SARS-CoV-2 Infections and Hospitalizations Among Persons Aged ≥16 Years, by Vaccination Status—Los Angeles County, California, May 1–July 25, 2021. Available at: cdc.gov/mmwr/volumes/70/wr/mm7034e5.htm?s_cid=mm7034e5_w. Accessed November 15, 2021.
- US Centers for Disease Control and Prevention. COVID-19 vaccine breakthrough case investigation and reporting. Available at: cdc.gov/vaccines/covid-19/health-departments/breakthrough-cases.html. Accessed November 15, 2021.
- Bakhshandeh B, Jahanafrooz Z, Abbasi A, et al. Mutations in SARS-CoV-2; Consequences in structure, function, and pathogenicity of the virus. Microb Pathog. 2021;154:104831.
From Dimensions of Dental Hygiene. December 2021;19(12)26-28,31.