-1001-/ISTOCK/GETTY IMAGES PLUS
-1001-/ISTOCK/GETTY IMAGES PLUS
A Preventive Approach to Molar Incisor Hypomineralization
This overview of molar incisor hypomineralization explores the clinical presentation and treatment of this developmental defect.
This course was published in the September 2021 issue and expires September 2024. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Discuss the enamel defect known as molar incisor hypomineralization (MIH), the teeth affected, and the minimal lesion size that should be recorded as MIH.
- Describe therapeutic options and treatment planning for patients with MIH, as well as long-term management strategies.
- Explain the use of glass ionomer and silver diamine fluoride when caring for this patient population.
Molar incisor hypomineralization (MIH) is a developmental and qualitative enamel defect caused by reduced mineralization and inorganic enamel components that lead to enamel discoloration and fractures of the affected teeth.1 This condition involves permanent first molars and incisors, and presents as demarcated lesions that range from creamy-white to yellow-brown opacities; it may also involve post-eruptive enamel breakdown and atypical restorations. The teeth might present with sensitivity. It occurs during the calcification stage of tooth formation, and is essentially a defect in enamel calcification. Unlike other enamel defects, hypomineralization starts at the enamel-dentinal junction and proceeds to the enamel’s surface. Mild lesions affect the inner enamel, while the entire enamel is affected in severe MIH. The affected enamel has 20% less mineral and three times to 15 times more protein than normal enamel.2
While its etiology is unknown, MIH is thought to be due to systemic and multifactorial disturbances during the last trimester of pregnancy up through the first 3 years of life.3 Influencing factors may include respiratory infections, low birth weight, fever, use of antibiotics, perinatal complications, dioxin, early childhood diseases, oxygen starvation, metabolic disorders, or prolonged breastfeeding.4,5 To be recorded as MIH, lesions must be larger than 1 mm. This condition has also been associated with primary second molars and the cusp tips of permanent canines.6 Hypomineralized second primary molars can be a predictor for MIH, but the absence of hypomineralized second primary molars does not rule out the appearance of MIH.7 These lesions should be differentiated from other enamel defects, such as fluorosis, amelogenesis imperfecta, enamel hypoplasia, white spot lesions, and Turner teeth (hypomineralization due to trauma).
The prevalence of MIH ranges from 2.4% to 40.2%.8 This wide range may be due to differences in standardization in identifying and recording MIH. Currently, one in six children is impacted worldwide.9 Affected molars are at a tenfold risk of developing caries when they are severely hypomineralized,10 and MIH teeth need five times to 10 times more dental treatment than teeth without MIH.6
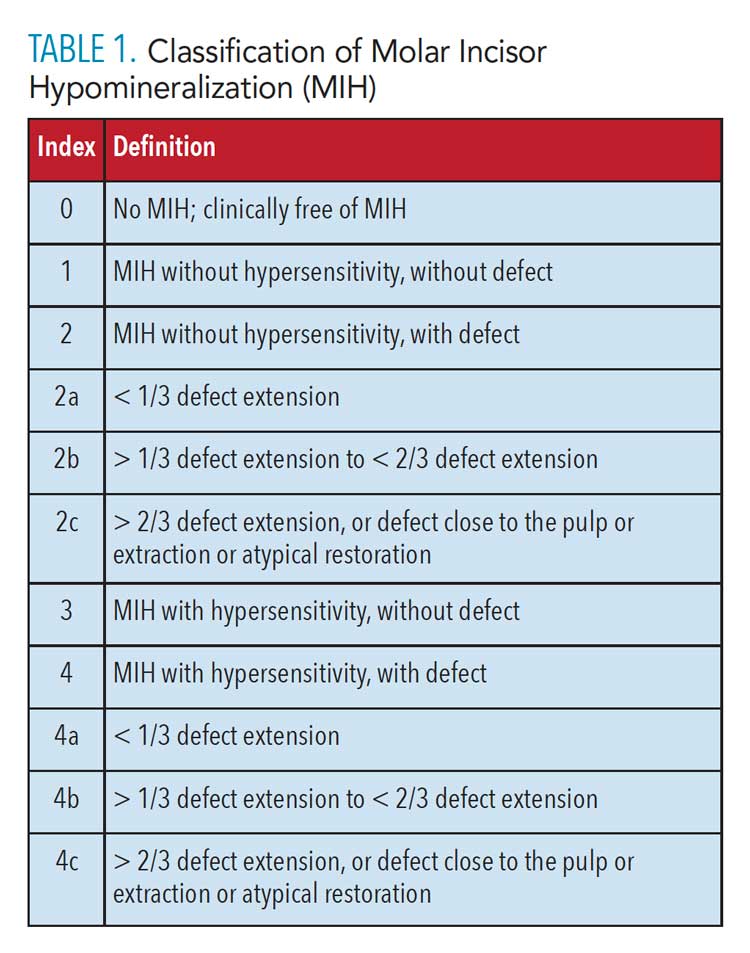
Classification of MIH is predicated on two chief considerations:
- Severity: mild, moderate, or severe11
- Treatment needs12
The classification system presented in Table 1 is based on hypersensitivity and the extent of enamel destruction; it could also be useful for epidemiological studies or assessment and treatment planning for individual patients.
![TABLE 1. Classification of Molar Incisor Hypomineralization (MIH)]() Differential Diagnosis
Differential Diagnosis
Clinicians should consider the following conditions when reaching a differential diagnosis for MIH:
Fluorosis. Mild types of fluorosis have diffused, poorly demarcated opacities along the perikymata and are caries resistant.13 Severe forms of fluorosis have yellow or brownish discoloration with pre-eruptive or post-eruptive breakdown and are susceptible to caries. The teeth affected are bilateral and symmetrical. Fluorosis occurs due to the exposure to excess of fluoride during the development of the enamel organ. It affects both primary and permanent teeth.14
Amelogenesis imperfecta. Affecting the structure and clinical appearance of enamel of all or almost all teeth, this condition is genetic in origin and has a family history. It may present with sensitivity and post-eruptive enamel breakdown. Found in both primary and permanent dentitions, there are four types of amelogenesis imperfecta: hypoplastic, hypomature, hypomineralized, and hypomature-hypoplastic.15,16
Enamel hypoplasia. This is a quantitative enamel defect with reduced enamel thickness. Post-eruptive teeth with enamel hypoplasia have smooth and regular surfaces. In comparison, MIH lesions with post-eruptive breakdown have sharp and irregular margins due to shearing of weakened enamel.2
White spot lesions. These usually manifest in areas of plaque accumulation by cervical areas of teeth. They are an early indication of caries and appear more chalky or opaque than adjacent enamel.
Turner teeth. This enamel defect in permanent teeth is associated with periapical infection in the overlying deciduous teeth. The affected teeth have a history of periapical infection or trauma to the overlying primary tooth. The lesion is limited to a single tooth with trauma and is asymmetrical.
Associated Concerns
The following problems are associated with MIH:2,17
- Affected teeth have a high risk of developing caries
- Teeth might be hypersensitive to thermal and mechanical stimuli, which may result in suboptimal brushing and subsequent plaque accumulation and rapid caries progression
- Severe MIH results in post-eruptive enamel breakdown, which will require extensive treatment
- Based on the severity of the affected teeth, a multidisciplinary approach might be necessary
- This condition can negatively impact the child’s quality of life
- Clinicians may have difficulty anesthetizing the tooth due to hypersensitive pulp tissue
- Esthetics is a concern if anterior teeth are affected
- Patient fear and anxiety are possible due to the need for multiple appointments
- Financial concerns for the patient’s parents/caregivers
Management Objectives
Adopting an enhanced preventive approach is crucial for patients with MIH. These individuals should be on a strict recare schedule that emphasizes early intervention. Affected teeth have a poor long-term prognosis, with high failure rates for restorations due to secondary caries and atypical restorations.18
Long-term treatment options include remineralization, sealants, resin infiltration, micro-abrasion, composites, veneers, and crowns.18 Hypersensitivity can be managed with arginine desensitizing toothpaste and fluoride varnish. As noted, additional local anesthetic procedures may be needed due to hypersensitive pulp tissue.2,18
For mild cases of MIH (which are mostly seen on incisors), a conservative approach may be appropriate and could include etching, bleaching, and sealing of the affected areas. For severe cases, micro-abrasion, veneers, or full-coverage crowns may be needed.
Teeth with MIH exhibit compromised bonding with adhesive restorations, such as sealants and composites, so the preparation should extend to sound tooth structure. Glass ionomer restorations can be used for temporization of teeth. Selective caries removal with high viscosity glass ionomer offers moderate survival rates.19 Amalgam has a high failure rate due to atypical-shaped restorations, which may need to be extended to achieve adequate retention. Direct restoration and use of sealants on MIH-affected teeth have three times greater need for retreatment than teeth without MIH.20
When first permanent molars (FPM) are severely affected and exhibit pain, extraction (with or without orthodontic alignment) may be considered before the eruption of second permanent molars (SPM). The ideal timing of extraction of FPMs is indicated radiographically by calcification of bifurcation of the roots on lower SPMs. If done at an ideal time, there is a 94% chance for ideal positioning of upper SPMs and 66% for lower SPMs after extraction of FPMs.21
Treatment Options
Considering the tooth structure is compromised and chances of secondary caries are high, the patient should be on strict recare and monitored regularly. The following treatment options can be considered for teeth affected by MIH:
Sealants. When placed on affected molars, sealants show survival rates similar to molars without MIH, suggesting this may be an effective preventive approach for MIH-affected molars.22 Resin-based sealants can be used if there is no evidence of enamel defects, hypersensitivity, or post-eruptive enamel breakdown. Glass ionomer sealants can be used to seal the fissures if any defects or hypersensitivity are present.9
Remineralization. Overwhelming evidence suggests the use of fluoride toothpaste reduces caries risk and remineralizes the surface layer. Use of products containing casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) and fluoride helps in remineralizing the body of the caries lesion,23–25 and calcium sodium phosphosilicate-containing toothpaste may aid remineralization while reducing sensitivity.26 Additionally, CPP-ACP helps saturate calcium and phosphate in the saliva to remineralize enamel and reduce sensitivity;27 however, CPP-ACP products should not be used in children with a history of allergy to milk products.
Bleaching. There is controversy over tooth bleaching in children younger than 18. Concerns for bleaching in adolescents include tooth sensitivity and gingival irritation. The General Dental Council revised its statement on using bleaching for patients younger than 18, stating, “Products containing or releasing between 0.1% and 6% hydrogen peroxide cannot be used on any person under 18 years of age, except where such use is intended wholly to treat or prevent disease.”28
Below are indications for bleaching in patients younger than 18:29
- Severe and moderate discoloration
- Enamel conditions
- White lesions, white markings, and white flecks
- Brown, orange, or yellow staining
- Coronal defects
- MIH
- Hereditary factors, such as dentinogenesis imperfect or amelogenesis imperfecta
- Traumatized/nonvital discolored anterior teeth
- Systemic diseases with oral health consequences (eg, diseases of the liver or kidneys, or hemorrhagic diseases)
Although tooth sensitivity is comparatively minor in adolescents compared to adults, preventing sensitivity is important for teeth with developmental defects. Consequently, clinicians may wish to consider prescribing a desensitizing toothpaste with silver nitrate for 2 weeks before bleaching treatment. Products such as CPP-ACP may also be beneficial. Sodium lauryl sulfate-containing products should not be used when bleaching due to the possibility of gingival irritation. If sensitivity is present, the frequency of the bleaching regimen should be reduced.
Carbamide peroxide is the preferred bleaching material, as its urea content acts as a cariostatic and antibacterial agent. Custom-fitted, vacuum-formed, nonreservoir trays made from 0.35-mm soft acrylic are recommended, as they minimize the amount of bleaching material needed. A nonscalloped tray design is acceptable, especially for soft tissues. Custom trays should be worn for a minimum of 2 hours under parental supervision. Carbamide peroxide is active for 10 hours, however, so overnight use is recommended for maximum benefit. Clinicians are advised that treatment should not be initiated if compliance is expected to be an issue.30
Resin infiltration. Also known as erosion infiltration, resin infiltration is a minimally invasive technique that uses low-viscosity resin to penetrate porous enamel due to caries or developmental defects. As noted, teeth affected with MIH present with opaque lesions with demarcated yellow or brown opacities. Even though mild MIH affects the enamel beneath the superficial two-thirds, studies have shown significant improvement in esthetics when resin infiltration is used on anterior teeth.31,32
Silver diamine fluoride (SDF). In 2014, the United States Food and Drug Administration approved SDF for use as a dental desensitizing agent, and in 2016, it granted breakthrough therapy status for treating severe early childhood caries.33 Once applied on a caries surface, SDF forms calcium fluoride and silver phosphate crystals. The fluoride reacts to the tooth structure, forming fluorapatite crystals that are highly insoluble in bacterial acids. The silver in silver phosphate provides antimicrobial properties.34 Upon application of SDF, the caries lesion turns black, and thus obtaining signed consent from the parent/caregiver is advised due to esthetic concerns.
Although severe reactions or pulpal damage have not been reported, SDF should not be placed on exposed pulps. In other situations, this agent has been successfully used for caries management in children and adolescents, including those with special healthcare needs.35 Additionally, SDF has not been shown to reduce the adhesion of resin or glass ionomer restorative materials.
Teeth affected with both MIH and hypersensitivity can be treated with the minimally invasive atraumatic restorative technique (ART) using 38% SDF and high-viscosity glass ionomer. This technique is known as silver-modified ART, or SMART.36
Conclusion
The result of a developmental defect that occurs during the calcification stage of tooth formation, MIH renders the enamel highly defective and prone to caries. Consequently, early diagnosis and intervention are expected to produce the most favorable outcomes. Treating the affected tooth or teeth can be challenging, however, due to increased sensitivity and discomfort. Application of sealants helps in prevention of caries lesions. Additionally, a fluoride-based toothpaste is recommended to aid remineralization of the tooth surface.
Other treatment approaches include bleaching and resin infiltration procedures on anterior teeth in patients with esthetic concerns. In cases in which esthetics is not a major concern, SDF can be applied on MIH-affected teeth to arrest caries and decrease hypersensitivity.
In all situations, the patient should be on strict recare with regular follow-up to identify any new caries lesions or failed restorations.
References
- Weerheijm KL, Duggal M, Mejare I, et al. Judgement criteria for molar incisor hypomineralisation (MIH) in epidemiological studies: a summary of the European meeting on MIH held in Athens, 2003. Eur J Pediatr Dent. 2003;4:110–113.
- Almuallem Z, Busuttil-Naudi A. Molar incisor hypomineralisation (MIH) — an overview. Br Dent J. 2018;225:601–609.
- Crombie F, Manton D, Kilpatrick N. Aetiology of molar-incisor hypomineralization: a critical review. Int J Pediatr Dent. 2009;19:73–83.
- Weerheijm KL. Molar incisor hypomineralization (MIH): clinical presentation, aetiology and management. Dental Update. 2004;31:9–12.
- Allazzam SM, Alaki SM, Meligy OA. Molar incisor hypomineralization, prevalence and etiology. Int J Dent. 2014;2014:234508.
- Lygidakis NA. Treatment modalities in children with teeth affected by molar-incisor enamel hypomineralisation (MIH): a systematic review. Eur Arch Pediatr Dent. 2010;11:65–74.
- Negre-Barber A, Montiel-Company JM, Boronat-Catalá M, Catalá-Pizarro M, Almerich-Silla JM. Hypomineralised second primary molars as predictor of molar incisor hypomineralisation. Sci Rep. 2016;6:319–329.
- Yannam SD, Amarlal D, Rekha CV. Prevalence of molar incisor hypomineralization in school children aged 8–12 years in Chennai. J Indian Soc Pedod Prev Dent. 2016;34:134–138.
- Ghanim A, Silva MJ, Elfrink ME, et al. Molar incisor hypomineralisation (MIH) training manual for clinical field surveys and practice. Eur Arch Paediatr Dent. 2017;18:225–242.
- Hubbard MJ. Molar hypomineralisation: what is the U.S. experience? J Am Dent Assoc. 2018;149:329–330.
- Mathi-Muju K, Wright JT. Diagnosis and treatment of molar incisor hypomineralization. Compend Contin Educ Dent. 2006;27:604–610.
- Steffen R, Kramer N, Bekes K. The Wurzburg MIH concept: the MIH treatment need index (MIH TNI). Eur Arch Paediatr Dent. 2017;18:355–361.
- Cameron AC, Widmer RP. Handbook of Pediatric Dentistry. 4th ed. Mosby: London; 2013.
- da Cunha Coelho AS, Mata PC, Lino CA, et al. Dental hypomineralization treatment: a systematic review. J Esthet Restor Dent. 2019;31:26–39.
- Witkop CJ Jr. Amelogenesis imperfecta, dentinogenesis imperfecta and dentin dysplasia revisited: problems in classification. J Oral Pathol. 1988;17:547–553.
- Crawford P, Aldred M, Bloch-Zupan A. Amelogenesis imperfecta. Orphanet J Rare Dis. 2007;2:17.
- Kalkani M, Balmer RC, Homer RM, Day PF, Duggal MS. Molar incisor hypomineralisation: experience and perceived challenges among dentists specializing in paediatirc dentistry and a group of general dental practitioners in the UK. Eur Arch Paediatr Dent. 2016;17:81–88.
- IAPD. Management of Molar Incisor Hypomineralization: Foundational Articles and Consensus Recommendations. 2020. Available at: http://www.iapdworld.org/07_ management-of-molar-incisor-hypomineralization. Accessed August 12, 2021.
- Durmus B, Sezer B, Tugcu N, Caliskan C, Bekiroglu N. Two-year survival of high-viscosity glass ionomer in children with molar incisor hypomineralization. Med Princ Pract. 2021;30:73–79.
- Kotsanos N, Kaklamanos EG, Arapostathis K. Treatment management of first permanent molars in children with molar-incisor hypomineralisation. Eur J Paediatr Dent. 2005;6:179–184.
- Teo TK, Ashley PF, Parekh S, Noar J. The evaluation of spontaneous space closure after the extraction of first permanent molars. Eur Arch Paediatr Dent. 2013;14:207–212.
- Fragelli CM, Souza JF, Bussaneli DG, Jeremias F, Santos-Pinto L, Cordeiro RC. Survival of sealants in molars affected by molar-incisor hypomineralization: 18-month follow up. Braz Oral Res. 2017;31:e30.
- Li J, Xie X, Wang Y, et al. Long-term remineralizing effect of casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) on early caries lesions in-vivo: a systematic review. J Dent. 2014;42:769–777.
- Shen P, Manton DJ. Cochrane NJ, et al. Effect of added calcium phosphate on enamel remineralization by fluoride in a randomized controlled in situ trial. J Dent. 2011;39:518–525.
- Al-Batayneh OB, Jbarat RA, Al-Khateeb SN. Effect of application sequence of fluoride and CPP-ACP on remineralization of white spot lesions in primary teeth: an in-vitro study. Arch Oral Biol. 2017;83:236–240.
- Abbasi Z, Bahrololoom M, Shariat M, Bagheri R. Bioactive glasses in dentistry: a review. J Dent Biomater. 2015;2:1–9.
- Di Marino JC. More Protection with Less Fluoride. Available at: https://www.premierdentalco.com/wp-content/uploads/2015/08/DiMarino_2015.pdf. Accessed August 12, 2021.
- General Dental Council. Position statement on tooth whitening. Available at: https://www.gdc-uk.org/docs/default-source/what-is-the-legal-position/tooth-whitening-position-statement.pdf. Accessed August 12, 2021.
- Greenwall L. Tooth Whitening Techniques. 2nd ed. Taylor and Francis: London; 2017.
- Greenwall-Cohen J, Greenwall L, Haywood V, Harley K. Tooth whitening for the under-18-year-old patient. Br Dent J. 2018;225:19–26.
- Bhandari R, Thakur S, Singhal P, Chauhan D, Jayam C, Jain T. Concealment effect of resin infiltration on incisor of grade I molar incisor hypomineralization patients: an in vivo study. J Conserv Dent. 2018;21:450–454.
- Mazur M, Westland S, Guerra F, et al. Objective and subjective aesthetic performance of icon treatment for enamel hypomineralization lesions in young adolescents: a retrospective single-center study. J Dent. 2018;68:104–108.
- Crystal YO, Niederman R. Evidence-based dentistry update on silver diamine fluoride. Dent Clin N Am. 2019;63:45–68.
- Shah S, Bhaskar V, Venkatraghavan K, Choudhary P, Ganesh M, Trivedi K. Silver diamine fluoride: a review and current applications. J Adv Oral Res. 2014;5:25–35.
- American Academy of Pediatric Dentistry. The Reference Manual of Pediatric Dentistry 2018–2019. 2018;40:152–161.
- MacLean J. Minimally invasive treatment for molar incisor hypomineralization. Decisions in Dentistry. 2018;4(11):18–20,22,23.
From Dimensions of Dental Hygiene. September 2021;19(9):36-39.