Post-Surgical Implant Care
Consistent maintenance that includes thorough removal of biofilm, cement, and calculus is critical to supporting long-term implant health.
Dental hygienists are key members of the oral care team in the post-treatment phase of implant therapy. The current scientific literature concludes that regular maintenance therapy with thorough debridement of biofilm and hard deposits is important for long-term implant health.1—9 In 2013, the American Academy of Periodontology (AAP) reported that 6% to 47% of implants show evidence of peri-implant mucositis or peri-implantitis.3 Professional maintenance appointment intervals should be at least one time to four times per year to ensure early detection and treatment of inflammation around implants.2,3,7 Jepsen et al10 found that 43.9% of patients with peri-implant mucositis who did not receive regular supportive care developed peri-implantitis, while only 18% of patients receiving consistent maintenance care progressed to peri-implantitis.

**Modification of Hempton Implant Maintenance Classifications from: Hempton TJ, Boncacci F, Lancaster D, Pechter J. Implant maintenance. Dimensions of Dental Hygiene. 2011;9(1):58—61.
Current choices for the subgingival debridement of implants include plastic, filled resin, titanium-coated metal, or pure titanium hand instruments; sonic or ultrasonic scalers with plastic or resin inserts/tips; rubber cup polishing; and glycine powder air polishing (GPAP). Before maintenance therapy begins, however, implant status must be assessed, as it is critical to effective dental hygiene treatment planning. While probing of implants has always been controversial, research demonstrates that gentle probing does not damage the peri-implant tissues1,4,11 and the use of a conventional metal probe may be acceptable on abutment surfaces.5,12
If signs of inflammation are present, careful probing should be performed using either a plastic or metal probe with light force to assess tissue tone, bleeding or suppuration, and probing depths. In addition to probing, the examination must include mobility testing, occlusion assessment, and the taking of radiographs to detect evidence of bone loss. Analysis of these factors will determine the appropriate classification of implant status as described by Hempton et al.13 Table 1 includes a classification system (modified from Hempton’s original) to guide clinicians in the selection of appropriate treatment modalities.13
CLASS I: HEALTHY TISSUE
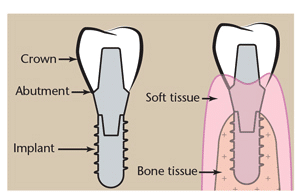
Healthy tissue around implants is firm, pink, does not bleed on probing, and is tightly adherent to the restoration. Well-maintained implants may have nonbleeding probing depths of ?5 mm, but they do not require aggressive or excessive subgingival instrumentation (Figure 1).
If the primary objective is biofilm removal, performing GPAP on implants provides effective bacteria removal with little or no alteration of the titanium surface.14—16 Glycine is an amino-acid salt that is extremely fine, gentle, and safe for use on titanium implant surfaces and restorative materials. Glycine powder can be used supragingivally with a regular air polishing device and tip. Glycine powder is delivered subgingivally with a unique thin tip on a specially designed air polisher that uses half the air pressure of a conventional air polisher. Several comparative in vitro studies found that GPAP was superior to plastic or titanium instruments and implant ultrasonic inserts/tips (UITs) in debriding biofilm from implant surfaces.17—20 GPAP does not, however, remove calculus or excess cement.
Rubber cup polishing with a nonabrasive toothpaste, tin oxide, or restorative polishing agent is an effective method for biofilm removal. Rubber cup polishing, however, may not extend as deep into the sulcus as subgingival air polishing, plastic or titanium hand instruments, or implant UITs.
Cleaning with floss or an implant oral hygiene cord may be helpful in removing biofilm during maintenance appointments. Also, some clinicians use the tip of a plastic probe or wooden toothpick tip secured to a handle to deplaque the sulcus with gentle, sweeping subgingival strokes.
CLASS II: PERI-IMPLANT MUCOSITIS
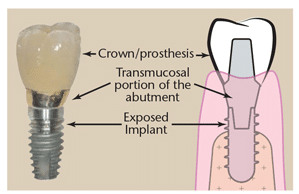
Peri-implant mucositis is the presence of inflamed tissue with bleeding on probing and ?5 mm probing depths but with no signs of bone loss. While the inflammation is generally a precursor to peri-implantitis, it can be resolved with thorough subgingival debridement of biofilm, calculus, and/or cement (Figure 2). Peri-implant mucositis is reversible when caught early.1—6,21,22

GPAP and rubber-cup polishing are effective for biofilm removal, but neither will remove calculus or excess cement. Plastic implant instruments cause the least alteration of the titanium implant surfaces; however, they are limited in their ability to adapt to implant configurations and remove hard deposits effectively and efficiently. The blades of plastic instruments are usually thicker than metal instruments in order to maintain their rigidity. When using plastic instruments, gently insert the blade subgingivally and use short, overlapping, horizontal, oblique, or vertical strokes around the implant restoration. More options have been introduced to the plastic instrument arena, including smaller, thinner universal curet designs such as the Barnhart 5/6.
Titanium implant instruments offer thinner blades than do plastic instruments. Studies have shown, however, that they may create varying degrees of alteration to the implant surface.23,24 Small titanium curets like the Barnhart 5/6 are available. The newest implant instruments include titanium Mini-bladed and Micro-mini-bladed Gracey curets that are easier to insert and adapt subgingivally due to their smaller working ends. Extreme caution must be taken when using titanium blades because they can damage the transmucosal seal and scratch the implant abutment.
Plastic hand instruments or plastic UITs cause the least amount of surface scratching compared to other types of implant instruments.1—7 One study demonstrated that carbon fiber- and glass fiber-reinforced plastic blades can significantly scratch smooth implant surfaces.24 Titanium instrument blades do not scratch implant surfaces as deeply as stainless steel, but they will cause damage if heavy pressure is applied.25 Stainless steel instrument blades cause the deepest scratches and are not recommended.1—6,25 Despite years of research showing in vitro and in vivo scratching of implant surfaces, literature reviews have concluded that roughened implant abutment surfaces caused by different instruments do not increase implant complications.3,26,27
A plastic UIT that is thin enough to insert easily under the tissue and extend to the base of the sulcus is an alternative choice. Use short, overlapping, horizontal, and oblique sweeping strokes to disrupt biofilm and flush out debris with water. Studies have shown no additional therapeutic effect from the addition of antimicrobials as irrigants during ultrasonic scaling of implants or natural teeth.22
If excess cement is present, do not attempt to remove it with implant instruments or implant UITs. Plastic and carbon fiber-reinforced plastic instruments are not effective for cement removal.28 Attempts to remove cement with titanium or stainless steel instruments often cause gouging and damage to the implant surface.3,6 The patient should be referred to the dentist who placed the restoration, a periodontist, or oral surgeon who can surgically expose the implant to gain access and remove the cement.1—3
CLASS III: PERI-IMPLANTITIS
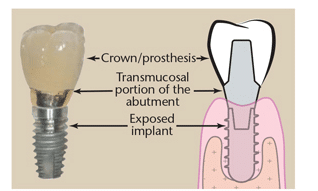
If peri-implantitis with severe inflammation, increased probing depths, bleeding on probing, suppuration, radiographic bone loss, exposed threads, calculus and/or cement, and mobility are present (Figure 3), referral to the dentist who placed the implant or a specialist is imperative. Mobility may be due to bone loss or loosening of the restoration. Peri-implantitis should be evaluated for contributory or secondary factors, such as residual cement and tobacco use, and proper interventions considered. Surgical intervention or endoscopic diagnosis and advanced treatment is required.1—3,29—31
Current literature indicates that nonsurgical periodontal therapy for peri-implantitis is not effective.1—3,21,27,32 If gingival recession allows easy access to the first or second exposed threads, successful nonsurgical treatment may be possible. Using a dental endoscope enables clinicians to view and more effectively remove subgingival cement and calculus from the implant, possibly increasing the success of nonsurgical therapy.29—31 Conventional scaling (without the aid of an endoscope) and irrigating failing implants are typically not enough to effectively treat peri-implantitis.1-4,21,22,26,32—34 Adjunctive therapies, such as subgingival irrigation or nonsurgical laser curettage, have limited or no significant long-term effects on peri-implantitis.1—6,33,35 Several studies found the adjunctive use of local delivery antibiotics, photodynamic therapy, and Er:YAG laser debridement produced short-term reduction of inflammation.4,26,29,32,33,36,38
Biofilm accumulation on residual cement as opposed to subgingival calculus is often the primary cause of peri-implantitis. Using a dental endoscope, Wilson29 found that excess dental cement was associated with 81% of peri-implant disease cases and removal of cement led to resolution of disease in 74% of these same cases. This excess cement usually is very tenacious and cannot be completely removed by conventional subgingival scaling. The use of screw-retained crowns rather than cemented crowns eliminates this problem.
If the patient is not referred for surgical treatment, progression to severe peri-implantitis with implant loss is likely.1—3 When an ailing implant can be viewed directly by flap surgery, laser surgery, or endoscopy, cement and/or calculus can be removed with hand or ultrasonic instruments, burs, or lasers.29—31 The contaminated titanium implant surfaces can also be air polished and treated topically with antimicrobials or antibiotics; systemic antibiotics also may be prescribed.
CLASS IV: FAILED IMPLANT
Implant failure is multifactorial and can occur early or late in the implant process. In addition to residual cement, some common factors are bacterial infection, improper surgical procedures, and occlusal overload. Suppuration and implant mobility with ongoing radiographic bone loss are classic signs of a failing implant (Figure 4).1—3 A referral to the dental implant surgeon is absolutely urgent for a patient with these symptoms.
Table 2. Hand Instruments Designed for Use on Implants*
*This list is designed to be as comprehensive as possible, but there may be inadvertent product omissions. |
CONCLUSION
Most hand and ultrasonic implant instruments are too large or too thick because they are most often copies of traditional instruments designed for natural teeth. The various configurations of implants, crowns, and prostheses pose unique challenges for subgingival instrumentation. Many hand and ultrasonic implant instruments do not adapt well and are too large or too flexible to remove calculus and/or cement effectively. Future research and development of new implant instrument designs are needed to improve clinical outcomes.
Effective implant maintenance is integral to the viability of this common therapy. While the rates of implant failure are low,3 clinicians need to remain up-to-date on advancements, such as dental endoscopy, GPAP, and new implant instrument designs, to ensure patients receive the best possible care.
REFERENCES
- Peri-implant mucositis and peri-implantitis: a current understanding of their diagnoses and clinical implications. J Periodontol. 2013 Apr;84:436—443.
- Cohen RE; Research, Science and Therapy Committee, American Academy of Periodontology. Position paper: periodontal maintenance. J Periodontol. 2003;74:1395—1401.
- Position Paper: Dental Implants in Periodontal Therapy. J Periodontol. 2000;71:1934—1942.
- Lindhe J, Meyle J; Group D of European Workshop on Periodontology. Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol. 2008;35(Suppl 8):282—285.
- Louropoulou A, Slot DE, Van der Weijden FA. Titanium surface alterations following the use of different mechanical instruments: a systematic review. Clin Oral Implants Res. 2012;23:643—658.
- Louropoulou A, Slot DE, Van der Weijden F. The effects of mechanical instruments on contaminated titanium dental implant surfaces: a systematic review. Clin Oral Impl Res. 2014;25:1149—1160
- Corbella S, Del Fabbro M, Taschieri S, De Siena F, Francetti L. Clinical evaluation of an implant maintenance protocol for the prevention of peri-implant diseases in patients treated with immediately loaded full-arch rehabilitations. Int J Dent Hyg. 2011;9:216—222.
- Grusovin MG, Coulthard P, Worthington HV, George P, Esposito M. Interventions for replacing missing teeth: maintaining and recovering soft tissue health around dental implants. Cochrane Database Syst Rev. 2010;8:CD003069.
- Hultin M, Komiyama A, Klinge B. Supportive therapy and the longevity of dental implants: a systematic review of the literature. Clin Oral Implants Res. 2007;18(Suppl 3):50—62.
- Jepsen S, Berglundh T, Genco R, et al. Primary prevention of peri-implantitis: managing peri-implant mucositis. J Clin Periodontol. 2015;42(Suppl 16):S152—S157.
- Heitz-Mayfield LJ. Peri-implant diseases: diagnosis and risk indicators. J Clin Periodontol. 2008;35(Suppl 8):292—304.
- Fakhravar B1, Khocht A, Jefferies SR, Suzuki JB. Probing and scaling instrumentation on implant abutment surfaces: an in vitro study. Implant Dent. 2012;21:311—316.
- Hempton TJ, Boncacci F, Lancaster D, Pechter J. Implant maintenance. Dimensions of Dental Hygiene. 2011;9(1):58—61.
- Schwarz F, Ferrari D, Popovski K, Hartig B, Becker J. Influence of different air-abrasive powders on cell viability at biologically contaminated titanium dental implants surfaces. J Biomed Mater Res B Appl Biomater. 2009;88:83—91.
- Sahm N, Becker J, Santel T, Schwarz F. Non-surgical treatment of peri-implantitis using an air-abrasive device or mechanical debridement and local application of chlorhexidine: a prospective, randomized, controlled clinical study. J Clin Periodontol. 2011;38:872—878
- Graumann SJ, Sensat ML, Stoltenberg JL. Air polishing: a review of current literature. J Dent Hyg. 2013;87:173—180.
- Mussano F, Rovasio S, Schierano G, Baldi I, Carossa S. The effect of glycine-powder airflow and hand instrumentation on peri implant soft tissues: a split-mouth pilot study. Int J Prosthodont. 2013;26;42—44.
- Sculean A, Bastendorf KD, Becker C, et al. A paradigm shift in mechanical biofilm management? Subgingival air polishing: a new way to improve mechanical biofilm management in the dental practice. Quintessence Int. 2013;44:475—477.
- Riben-Grundstrom C, Norderyd O, André U, Renvert S. Treatment of peri-implant mucositis using a glycine powder air-polishing or ultrasonic device. A randomized clinical trial. J Clin Periodontol. 2015;42:462—469.
- De Siena F, Corbella S, Taschieri S, Del Fabbro M, Francetti L. Adjunctive glycine powder air-polishing for the treatment of peri-implant mucositis: an observational clinical trial. Int J Dent Hyg. 2014. Epub ahead of print.
- Romanos GE, Weitz D. Therapy of peri-implant diseases. Where is the evidence? J Evid Based Dent Pract. 2012;12(Suppl 3):204—208.
- Matthews DC. Prevention and Treatment of Periodontal Diseases in Primary Care. Evid Based Dent. 2014;15:68—69.
- Dmytryk JJ, Fox SC, Moriarty JD. The effects of scaling titanium implant surfaces with metal and plastic instruments on cell attachment. J Periodontol. 1990;61:491—496.
- Hasturk H, Nguyen DH, Sherzai H, et al. Comparison of the impact of scaler material composition on polished titanium implant abutment surfaces. J Dent Hyg. 2013;87:200—211.
- Matarasso S, Quaremba G, Coraggio F, Vaia E, Cafiero C, Lang NP. Maintenance of implants: an in vitro study of titanium implant surface modifications subsequent to the application of different prophylaxis procedures. Clin Oral Implants Res. 1996;7:64—72.
- Esposito M, Grusovin MG, Worthington HV. Interventions for replacing missing teeth: treatment of peri-implantitis. Cochrane Database Syst Rev. 2012;18:CD004970.
- Persson GR, Roos-Jansåker AM, Lindahl C, Renvert S. Microbiologic results after non-surgical erbium-doped:yttrium, aluminum, and garnet laser or air-abrasive treatment of peri-implantitis: a randomized clinical trial. J Periodontol. 2011;82:1267—1278.
- Schmage P, Kahili F, Nergiz I, Scorziello TM, Platzer U, Pfeiffer P. Cleaning effectiveness of implant prophylaxis instruments. Int J Oral Maxillofac Implants. 2014;29:331—337.
- Wilson TG Jr. The positive relationship between excess cement and peri-implant disease: a prospective clinical endoscopic study. J Periodontol. 2009;80:1388—1392.
- Wilson TG Jr, Valderrama P, Rodrigues DB. The Case for Routine Maintenance of Dental Implants. J Periodontol. 2014;85:657—660.
- Wilson TG. The Use of the Dental Endoscope and Videoscope for Diagnosis and Treatment of Peri-Implant Diseases. In: Minimally Invasive Periodontal Therapy: Clinical Techniques and Visualization Technology. SK Harrel and TG Wilson, eds. John Wiley & Sons, Inc, 2015: Hoboken, NJ.
- Renvert S, Polyzois I, Persson GR. Treatment modalities for peri-implant mucositis and peri-implantitis. Am J Dent. 2013;26:313—318.
- Renvert S, Roos-Jansåker AM, Claffey N. Non-surgical treatment of peri-implant mucositis and peri-implantitis: a literature review. J Clin Periodontol. 2008;35(Suppl 8):305—315.
- Smeets R, Henningsen A, Jung O, Heiland M, Hammächer C, Stein JM. Definition, etiology, prevention and treatment of peri-implantitis–a review. Head Face Med. 2014;10:34.
- Schwarz F, Sculean A, Rothamel D, Schwenzer K, Georg T, Becker J. Clinical evaluation of an Er:YAG laser for nonsurgical treatment of peri-implantitis: a pilot study. Clin Oral Impl Res. 2005;16:44—52.
- Bassetti M, Schär D, Wicki B, et al. Anti-infective therapy of peri-implantitis with adjunctive local drug delivery or photodynamic therapy: 12-month outcomes of a randomized controlled clinical trial. Clin Oral Implants Res. 2014;25:279—287.
- Salvi GE, Persson GR, Heitz-Mayfield LJ, Frei M, Lang NP. Adjunctive local antibiotic therapy in the treatment of peri-implantitis II: clinical and radiographic outcomes. Clin Oral Implants Res. 2007;18:281—285.
- Renvert S, Polyzois IN. Clinical approaches to treat peri-implant mucositis and peri-implantitis. Periodontol 2000. 2015;68:369—404.
From Dimensions of Dental Hygiene. May 2015;13(3):24,26,28—29.