Periodontal Disease Management
Understanding the biology behind periodontal disease phenotypes will help oral health professionals create individualized periodontal treatment plans.
This course was published in the July 2018 issue and July 31, 2021. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Explain the concept of clinical phenotypes of periodontal disease.
- Discuss the etiology of periodontal disease.
- Identify methods to help patients prevent periodontal disease.
Today, understanding the biology underlying different clinical phenotypes of periodontal disease should aid oral health professionals in establishing periodontal prognosis and developing novel and more personalized periodontal treatments.8
Current periodontal classifications have been updated to alleviate the clinical challenge of classifying periodontal conditions primarily based on attachment loss and microbiological test results, which can be sometimes ambiguous.9 Baelum and Lopez10 stated that “periodontal disease is a syndrome that comes in all sizes,” suggesting that there are no clear demarcations between health and disease or between conditions. It appears now that it is the initial and early host-inflammatory and immune responses to the bacterial assault that seem to favor the emergence of the classic periopathogenes and which determine periodontal disease development or progression.8
Recent evidence-based concepts reveal inflammation is critical in diseases like cardiovascular disease and Alzheimer’s disease, which previously have not been considered inflammatory.11 Today, inflammatory mechanisms are recognized as integral to the development and progression of most chronic diseases of aging.8,11–13 Environmental and genetic variations interact and account for differences in inflammation among individuals.11 While inflammation in the body is actively resolved by protective mechanisms that help to restore homeostasis, there are ways to support these processes.11,14 Although genes do not change, gene expression in specific tissues can evolve significantly throughout life by factors such as diet, stress, and bacterial accumulations.15 Abdominal and visceral fat accumulations increase the inflammatory burden on the body,12 and overexpression of inflammation may be one of the key aspects of aging that influences and links various diseases in individuals.14,16
Kornman17 and others assert that chronic adult periodontitis is a bacterially induced chronic inflammatory disease that destroys the attachment apparatus supporting the teeth.5 Experimental evidence implicates bacterial plaque as the primary etiological factor for initiating periodontitis. Concomitantly, specific periopathogenic bacteria and immune-inflammatory mechanisms are involved in periodontitis in complex interactions.18
In the model of periodontal pathogenesis including this concept, the bacteria activate an immune-inflammatory mechanism. This adversely affects the host control of the bacterial challenge, as well as the remodeling of osseous and gingival structures.17 Various environmental and genetic factors are changing the clinical appearance of periodontal disease in a patient.17
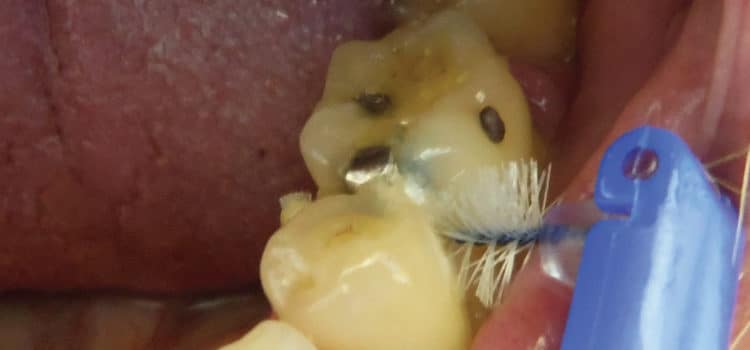
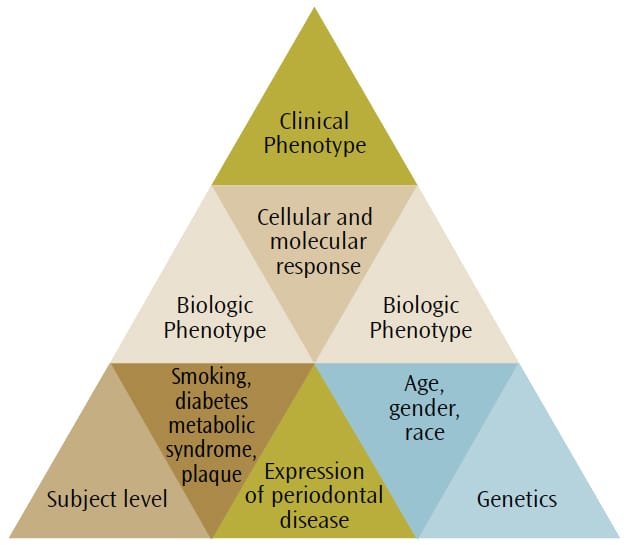
Any clinical phenotype of periodontal disease can be broken down into respective subclinical components to provide a “biologic systems model” for classifying periodontal disease in humans (Figure 1).19 In disease, the biologic systems model incorporate all components that contribute to the final clinical presentation of disease.4,8 The outermost factors are the individual exposures of each subject, including the characteristics of his or her own dental biofilm (eg, the type of bacterial infection), as well as the presence of medical conditions (eg, diabetes and obesity) and environmental influences (eg, smoking). The subject-level component interacts with the genetic background, as the evidence suggests that specific gene polymorphisms are associated with periodontal disease,14influencing the host response to infections.20 Figure 2. The patient is using an interdental brush in the lower posterior region. The lingual approach is recommended if the buccal embrasure is too narrow to fit the brush head through comfortably. As a rule, the bigger the brush that can be inserted between the teeth without trauma, the more effective the cleaning is. Dipping the interdental brush in antimicrobial mouthrinse before insertion will not only disrupt the interdental and subgingival biofilm, but also reduce the overall bacterial load in the mouth.
ETIOLOGY AND PREVENTION OF PERIODONTAL DISEASE
In more than half a century of worldwide translational research and clinical studies, it has been firmly established that effective and persistent removal of dental plaque is conditional to achievement and maintenance of oral health.21 According to the nonspecific plaque hypothesis, any reduction of the noxious load of plaque and its bacterial byproducts adjacent to gingiva will reduce gingivitis. Gingivitis is the precursor of periodontal disease. As supragingival plaque matures and progresses to subgingival plaque, any decrease in bacterial deposits will prevent gingivitis. The maintenance of noninflamed gingiva will help prevent chronic periodontitis. As the dental biofilm accumulates, oral hygiene helps to control the amount of bacterial deposits on the dental structures and their apical migration. This constitutes primary gingivitis prevention.22
While it is generally recommended that patients remove the dental plaque by brushing their teeth at least twice per day, it is evident from epidemiological and clinical studies that home-based mechanical oral hygiene procedures, such as brushing and flossing performed by most patients, are not sufficient to reduce plaque buildup and prevent disease.23–25 Although no statistically significant difference was found between power and manual toothbrushes,26 rotating and oscillating brushes were shown to reduce short- and long-term biofilm formation and gingival disease. Another advantage to power toothbrushes is the timer function that indicates length of brushing, helping patients reach the recommended 2 minutes. Overall, patients tend to use less pressure when brushing with a power toothbrush due to pressure sensors within the brush. This leads to less trauma to the buccal gingiva, possibly reducing the risk of recession.27
According to research, bisbiguanide compounds, like chlorhexidine gluconate and alexidine, are the most effective mouthrinse ingredients used to inhibit plaque formation, especially after mechanical removal of the biofilm. Chlorhexidine has shown less plaque inhibition when included in dentifrices. This is probably due to flavor, detergent, and blood inactivating the antimicrobial inhibitory pathways.28,29 Mouthrinses containing essential oils (thymol, menthol, eucalyptol, and methyl salicylate), as well as those with cetylpyridinium chloride have been shown to be effective in decreasing supragingival plaque and gingivitis.30,31 While not an antiseptic, mouthrinse containing delmopinol helps to prevent plaque from adhering to the teeth and gingiva.32
Some oral hygiene agents have side effects. Chlorhexidine can cause external staining of teeth.33 Pyrophosphates, flavorings, and detergents, such as sodium lauryl sulfate, have been implicated as possible causative factors in specific mucosal and gingival hypersensitivities (eg, aphthous ulcers, stomatitis, cheilitis, burning mouth syndrome, and oral mucosal desquamation).33–35 Oral health professionals should recommend alternatives if this is the case.
Prevention is based on the principle that the greatest effect is achieved where the greatest risk is identified.36 Periodontal lesions in patients with increased risk for periodontal disease are located interdentally rather than buccally or lingually, as cleaning of tooth surfaces is facilitated by the ability of the toothbrush to access the location. The anatomic interproximal gingival structure under the contact point of adjacent teeth, or the col, is not keratinized. It is also the area of maximum plaque accumulation and is the most difficult to access.
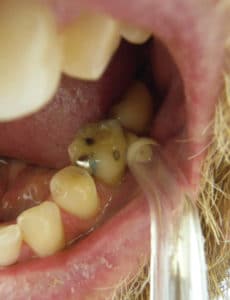
While patients may have an interdental aid preference,37 factors such as gingival biotype; anatomy of the embrasure between teeth; tooth location in the dental arch and alignment; and patient’s dexterity, motivation, and compliance should be considered when giving interproximal oral hygiene instructions.38–41 Most studies show improvement of plaque scores when comparing patients using interproximal brushes with the use of floss.39 Two of three studies showed that the regular use of interdental brushes reduced pocket depth more than the use of floss in patients with periodontitis.18,42 One study compared interdental brushes with wood sticks and found the interproximal brushes to be more effective in plaque removal (Figure 2, page 42).42 The most effective cleaning is achieved when the biggest brush size that fits into the embrasure is used. As such, patients need to have different brush sizes available to use for anterior teeth and posterior areas. In areas of dental crowding and in young patients where the papillae fill out the embrasures, dental floss is suitable for interdental oral hygiene.23
Three studies found a reduction in inflammatory response when adding oral irrigation to a self-care regimen.29,42,43 In two studies, pocket depth reduction was also achieved (Figure 3, page 43).29,43 Accessories for oral irrigator devices facilitate up to 90% penetration of the irrigation liquid into a 6 mm pocket when the tip is inserted 1 mm subgingivally.44 Oral irrigation potentially induces bacteremia, when compared with toothbrushing,45,46 interdental flossing,47,48 scaling and root planning,49 and even simple chewing,50 because the gingiva is subjected to considerable force during even supragingival irrigation. For healthy patients, however, the literature attests to the safety of oral irrigation.
The clinical effects of oral irrigation are multifold. When used daily supragingivally, oral irrigation alters the composition of periodontal pathogens and their interaction with the host response in patients with gingivitis.43 The water pulsation may reduce inflammation independently from biofilm debridement.51In any case, flushing out macroscopic debris and bacterial components in pathogenic floating plaque interferes with the maturation of the plaque biofilm and may even stimulate the host immune response.42 The discrepancy between the positive effect of irrigation in reducing gingival inflammation and the statistical significance of its effect in the literature may be related to research using an all-or-nothing grading system, in which plaque thickness is not considered.52,53
In conclusion, the research asserts that one single oral hygiene instruction by a dental professional can positively affect the prognosis of periodontitis in a patient for about 6 months afterward. This is in congruence with the 3-month to 6-month recare interval.23,27,36This confirms the importance of patient education by oral health professionals in the prevention of periodontal disease.
Acknowledgement The authors would like to thank Christopher Aloise, BA; Marc Rabins, BA, BS; and Samuel Rabins for their help with this manuscript.
REFERENCES
- Armitage GC. Periodontal diagnoses and classification of periodontal diseases. Periodontol 2000. 2004;34:9–21.
- Hajishengallis G. The inflammophilic character of the periodontitis-associated microbiota. Mol Oral Microbiol.2014;29:248–257.
- American Academy of Periodontology. Parameters of care. J Periodontol. 2000;71(5 Suppl):847–883.
- Kowalski J, Górska R. Clinical and microbiological evaluation of biofilm-gingival interface classification in patients with generalized forms of periodontitis. Pol J Microbiol. 2014;63:175–181.
- Sanz M, van Winkelhoff AJ; Working Group 1 of Seventh European Workshop on Periodontology. Periodontal infections: understanding the complexity–consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol.2011;38(Suppl 11):3–6.
- Offenbacher S, Barros SP, Singer RE, Moss K, Williams RC, Beck JD. Periodontal disease at the biofilm-gingival interface. J Periodontol. 2007;78:1911–1925.
- Chen B, Wu W, Sun W, Zhang Q, Yan F, Xiao Y. RANKL expression in periodontal disease: where does RANKL come from? Biomed Res Int. 2014;2014:731039.
- Bartold PM, Van Dyke TE. Periodontitis: a host-mediated disruption of microbial homeostasis. Unlearning learned concepts. Periodontol 2000. 2013;62:203–217.
- American Academy of Periodontology Task Force Report on the Update to the 1999 Classification of Periodontal Diseases and Conditions. J Periodontol. 2015;86:835–838.
- Baelum V, Lopez R. Defining and classifying periodontitis: Need for a paradigm shift? Eur J Oral Sci. 2003;111:2–6.
- Beader N, Ivić-Kardum M. The role of cytomegalovirus infection in the pathogenesis of periodontal diseases. Acta Clin Croat. 2011;50:61–66.
- Cavalcante-Silva LH, Galvão JG, da Silva JS, de Sales-Neto JM, Rodrigues-Mascarenhas S. Obesity-driven gut microbiota inflammatory pathways to metabolic syndrome. Front Physiol. 2015;6:341.
- Miklossy J, McGeer PL. Common mechanisms involved in Alzheimer’s disease and type 2 diabetes: a key role of chronic bacterial infection and inflammation. Aging (Albany NY). 2016;8:575–588.
- Yousuf O, Mohanty BD, Martin SS, et al. High-sensitivity C-reactive protein and cardiovascular disease: a resolute belief or an elusive link? J Am Coll Cardiol. 2013;62:397–408.
- Divaris K, Monda KL, North KE, et al. Exploring the genetic basis of chronic periodontitis: a genome-wide association study. Hum Mol Genet. 2013;22:2312–2324.
- Sardi F, Fassina L, Venturini L, et al. Alzheimer’s disease, autoimmunity and inflammation. The good, the bad and the ugly. Autoimmun Rev. 2011;11:149–153.
- Kornman KS. Mapping the pathogenesis of periodontitis: a new look. Periodontol. 2008;79(8 Suppl):1560–1568.
- Herbert BA, Novince CM, Kirkwood KL. Aggregatibacter actinomycetemcomitans, a potent immunoregulator of the periodontal host defense system and alveolar bone homeostasis. Mol Oral Microbiol. 2016;31:207–227.
- Hertzog PJ, Mansell A, van Driel IR, Hartland EL. Sculpting the immune response to infection. Nat Immunol.2011;12:579–582.
- Zhang J, Sun X, Xiao L, Xie C, Xuan D, Luo G. Gene polymorphisms and periodontitis. Periodontol 2000. 2011;56:102–124.
- Preshaw PM. Detection and diagnosis of periodontal conditions amenable to prevention. BMC Oral Health. 2015;15(Suppl 1):S5.
- Dehghani M, Abtahi M, Sadeghian H, Shafaee H, Tanbakuchi B. Combined chlorhexidine-sodiumfluoride mouthrinse for orthodontic patients: Clinical and microbiological study. J Clin Exp Dent. 2015;7:e569–575.
- Van Der Weijden F, Slot DE. Oral hygiene in the prevention of periodontal diseases: the evidence. Periodontol 2000. 2011;55:104–123.
- Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of severe periodontitis in 1990-2010: a systematic review and meta-regression. J Dent Res. 2014;93:1045–1053.
- Nightingale KJ, Chinta SK, Agarwal P, et al. Toothbrush efficacy for plaque removal. Int J Dent Hyg. 2014;12:251–256.
- Jain Y. A comparison of the efficacy of powered and manual toothbrushes in controlling plaque and gingivitis: a clinical study. Clin Cosmet Investig Dent. 2013;5:3–9.
- Sanz M, Bäumer A, Buduneli N, et al. Effect of professional mechanical plaque removal on secondary prevention of periodontitis and the complications of gingival and periodontal preventive measures: consensus report of group 4 of the 11th European Workshop on Periodontology on effective prevention of periodontal and peri-implant diseases. J Clin Periodontol. 2015;42(Suppl 16):S214–S220.
- Marrelli M, Amantea M, Tatullo M. A comparative, randomized, controlled study on clinical efficacy and dental staining reduction of a mouthwash containing Chlorhexidine 0.20% and Anti Discoloration System (ADS). Ann Stomatol (Roma). 2015;6:35–42.
- James P, Worthington HV, Parnell C, et al. Chlorhexidine mouthrinse as an adjunctive treatment for gingival health. Cochrane Database Syst Rev. 2017;31:CD008676.
- Bauroth K, Charles C , Mankodi S, Simmons D, Zhao Q, Kumar L. The efficacy of an essential oil antiseptic mouthrinse vs. dental floss in controlling interproximal gingivitis. J Am Dent Assoc. 2003;134:359–365.
- Mankodi S, Bauroth K, Witt JJ, et al. A 6-month clinical trial to study the effects of a cetylpyridinium chloride mouth rinse on gingivitis and plaque. Am J Dent. 2005;18(Special Issue):9A–14A.
- Stoeken JE, Paraskevas S, van der Weijden GA. The long-term effect of a mouthrinse containing essential oils on dental plaque and gingivitis: a systematic review. J Periodontol. 2007;78:1218–1228.
- Dadkhah M, Chung NE, Ajdaharian J, Wink C, Klokkevold P, Wilder-Smith P. Effects of a novel dental gel on plaque and gingivitis: a comparative study. Dentistry (Sunnyvale). 2014 Jun 1;4(6):239.
- Ghapanchi J, Kamali F, Moattari A, et al. In vitro comparison of cytotoxic and antibacterial effects of 16 commercial toothpastes. J Int Oral Health. 2015;7:39–43.
- Lawrence LM, Farquharson A, Brown RS, Vatanka HO. Oral tissue irritants in toothpaste: a case report. J Clin Pediatr Dent. 2013;38:75–78.
- Prasad RV, Chincholi S, VD, et al. Iatrogenic factors affecting the periodontium: an overview. Open Dent J. 201526;9:208–209.
- van der Weijden F, Slot DE. Oral hygiene in the prevention of periodontal diseases: the evidence. Periodontol 2000. 2011;55:104–123.
- Mariotti A, Hefti AF. Defining periodontal health. BMC Oral Health. 2015;15(Suppl 10:S6.
- Ercan N, Erdemir EO, Ozkan SY, Hendek MK. The comparative effect of propolis in two different vehicles; mouthwash and chewing-gum on plaque accumulation and gingival inflammation. Eur J Dent. 2015;9:272–276.
- Tariq M, Iqbal Z, Ali J, et al. Treatment modalities and evaluation models for periodontitis. Int J Pharm Investig. 2012;2:106–122.
- Poklepovic T, Worthington HV, Johnson TM, et al. Interdental brushing for the prevention and control of periodontal diseases and dental caries in adults. Cochrane Database Syst Rev. 2013;12:CD009857.
- Sambunjak D, Nickerson JW, Poklepovic T, et al. Flossing for the management of periodontal diseases and dental caries in adults. Cochrane Database Syst Rev. 2011;(12):CD008829.
- Jones CG. Chlorhexidine: is it still the gold standard? Periodontol 2000. 1997;15:55–62.
- Tonetti MS, Lang NP, Cortellini P, et al. Effects of a single topical doxycycline administration adjunctive to mechanical debridement in patients with persistent/recurrent periodontitis but acceptable oral hygiene during supportive periodontal therapy. J Clin Periodontol. 2012;39:475–482.
- Kerpen SJ. Periodontitis and the heart. J Am Dent Assoc. 2012;143:1182.
- Kimura K, Takase B. Significant association between periodontitis and cardiovascular risk. Circ J. 2014;78:837–838.
- Tomás I, Diz P, Tobías A, Scully C, Donos N. Periodontal health status and bacteraemia from daily oral activities: systematic review/meta-analysis. J Clin Periodontol. 2012;39:213–228.
- Hamers LA, Linssen CF, Lancé MD, , et al. Positive cultures from cardiopulmonary bypass: prevalence and relevance regarding postoperative infection. Eur J Cardiothorac Surg. 2011;40:372–378.
- Odum SM, Fehring TK, Lombardi AV, et al. Periprosthetic Infection Consortium.Irrigation and debridement for periprosthetic infections: does the organism matter? J Arthroplasty. 2011;26(6 Suppl):114–118.
- Waghmare AS, Vhanmane PB, Savitha B, Chawla RL, Bagde HS. Bacteremia following scaling and root planing: A clinico-microbiological study. J Indian Soc Periodontol. 2013;17:725–730.
- Van Strydonck DA, Slot DE, Van der Velden U, Van der Weijden F. Effect of a chlorhexidine mouthrinse on plaque, gingival inflammation and staining in gingivitis patients: a systematic review. J Clin Periodontol. 2012;39:1042–1055.
- Nagarakanti S, Gunupati S, Chava VK, Reddy BV. Effectiveness of subgingival irrigation as an adjunct to scaling and root planing in the treatment of chronic periodontitis: a systematic review. J Clin Diagn Res. 2015;9:e6–9.
- James P, Worthington HV, Parnell C, et al. Chlorhexidine mouthrinse as an adjunctive treatment for gingival health. Cochrane Database Syst Rev. 2017;3:CD008676.
From Dimensions of Dental Hygiene. July 2018;16(7):42-45.




[…] Scientists from the National Institute of Dental and Craniofacial Research (NIDCR) and the University of Pennsylvania School of Dental Medicine have identified cells as therapeutic targets in the treatment of periodontitis. […]
[…] researchers found oral P. gingivalis infection led to brain colonization in a murine model of periodontal disease. The bacterium also increased production of amyloid beta (Aβ), a component of the amyloid plaques […]