
Noncarious Cervical Lesions
Dental hygienists play an important role in the diagnosis and management of these lesions.
Noncarious cervical lesions (NCCLs) are defined as a loss of hard dental tissue near the cementoenamel junction, usually on the buccal surfaces of teeth, resulting in a grooved or wedge-shaped area of missing tooth structure. These lesions are increasing in prevalence, especially among adolescents and older adults.1 In particular, lifestyle changes—including the increased consumption of acidic drinks among young people and the high number of older adults who take prescription medications, which can cause hyposalivation and xerostomia—raise the risk of NCCLs. Hyposalivation increases acidity in the oral cavity, softening the tooth surface and facilitating tooth loss (Figure 1).
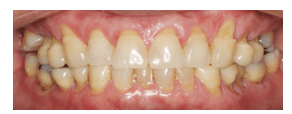
A recent study reported that the prevalence of NCCLs in teenagers and adults ranges from 11% to 62%, and the number of lesions increases with age.2 Lesions most often occur on the buccal of maxillary premolars, but are also commonly found on other maxillary and mandibular anterior and premolar teeth.
ETIOLOGY
The exact etiology of NCCLs remains unknown, but it is generally accepted that their cause is multifactorial.1,3,4 The clinical endpoint of NCCLs is the wearing away of the teeth. The mechanisms involved include acid erosion (loss of tooth structure by acid demineralization without the involvement of bacteria); abrasion (friction resulting in loss of tooth structure from toothbrushing, dentifrices, etc); and abfraction (breaking away of tooth structure due to tensile stresses in the cervical area).1,3,4
Acid erosion is the most important factor involved in the development of NCCLs. Saliva normally keeps the oral environment in the 7.0 pH range (neutral) due to its ability to buffer acids. The source of acids can be intrinsic (bulimia, gastroesophageal reflux disease [GERD]) or extrinsic (fruit juices, sports and energy drinks, and wine, to name a few). When oral acids bring the pH of the tooth surface below 5.5, demineralization of enamel and dentin can begin.5
Demineralization causes the loss of calcium (inorganic matter) from the tooth surface, followed by a softening of the top layers of dentin and enamel. This softened layer then becomes susceptible to toothbrush abrasion, which can cause wedge-shaped cervical lesions. The thin, softened layer is between 0.2 μm and 3 μm thick and is repeatedly removed by toothbrush abrasion—leading to permanent loss of tooth volume in the cervical area.6
Both the pH (less than 5.5) and the strength (buffering capacity) of the acid challenge need to be considered. For example, Red Bull Energy Drink (citric acid) is stronger than Coca Cola (phosphoric acid). Regular and diet sodas and sports and energy drinks range from a pH of 2.53 to 3.4, respectively.7 Lemon, grapefruit, and orange juices also cause erosive damage to enamel. Gastric acids from GERD are the most erosive, with a pH of 1.0 to 2.0.1
Saliva plays an important role—cleaning, diluting, and buffering acids. Patients with xerostomia are much more susceptible to acid erosion. As it takes 30 minutes for saliva to return to normal after an acid challenge is experienced, as well as for the teeth to remineralize, patients should be advised to avoid brushing immediately after an acid exposure.8
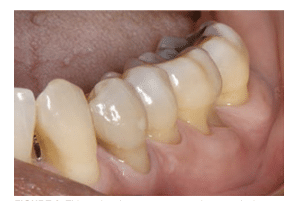
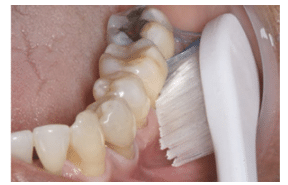
Dental abrasion—another contributing factor to NCCLs—is defined as wearing away of dental hard tissues by frictional forces.1 This can include wear caused by toothbrushing, flossing, tongue action, and rubbing from opposing surfaces that are hard or rough, such as unpolished porcelain crowns. The effects of such actions are usually more pronounced on dentin surfaces than on enamel surfaces (Figure 2 and Figure 3).9
It appears that toothbrushing alone—regardless of the brush stiffness, filament size, aggressiveness, or the frequency of brushing—causes little abrasive tooth wear.1 Rather, it is the abrasiveness of the toothpaste that is responsible for the loss of tooth structure.9,10 In general, popular brands have very low abrasivity. Dentifrices that include “whitening agents” or promote their ability to provide “tartar control” tend to be more abrasive. Toothbrushes with stiffer bristles have also been linked to increased gingival recession.11
Abfraction is the microstructural loss of tooth structure due to excessive occlusal forces transmitted to the cervical area as flexural forces.1 These flexural forces, usually caused by cyclic loading, lead to the breaking away of enamel rods in the cervical region of the tooth—causing microfractures of cementum and dentin.4 Bruxism may also contribute to the problem.12
Another possible modifying factor in abfraction formation involves the mobility of teeth and the effects of occlusal stresses in the formation of cervical lesions. A relationship has been found between tooth cervical lesions, tooth stability, and periodontal support. Thus, the more mobile the teeth, the less susceptible they are to excessive occlusal forces. The teeth move under occlusal loads, resulting in less concentration of forces in the cervical areas.13
While these interocclusal relationships may be contributing factors in the formation of some NCCLs, it is likely that clinical signs of abfraction in cervical areas are not simply related to stress concentrations. Rather, they may play a role, along with acid erosion and toothbrush abrasion, in the multifactorial etiology of NCCLs.1,4
MANAGEMENT
NCCLs can occur at any time and may worsen throughout the lifespan. Without intervention, they can result in tooth fracture, hypersensitivity, and cosmetic problems. Therefore, early diagnosis is important in order to start behavioral changes and to avoid future invasive and expensive restorative interventions. Dental hygienists play a critical role in the recognition of these problems.
Clinical management of NCCLs includes first identifying the various etiologic factors present in a given patient. For example, if a patient is an active bruxer, has wear facets on the teeth, and presents with NCCLs, the dentist may make adjustments to remove occlusal interferences and/or prescribe an occlusal splint or night guard.4
Through the process of taking a comprehensive medical history, dental hygienists should question patients regarding their intake of acidic foods or beverages, including fruits, wine, and sports and energy drinks. It is important to monitor the frequency, duration, sequencing, and the mode of intake of these drinks. For example, the sipping and swishing of a drink is much more erosive than gulping the beverage.14 Consuming dairy after an acid challenge will help raise the pH and remineralize the teeth.15 Medication use should be reviewed, as drug-related xerostomia can contribute to tooth loss. Finally, questions regarding GERD should be asked, because there is a positive correlation between this disorder and dental erosion.16 These medical and behavioral factors have the net, and possibly cumulative, effect of causing demineralization of the tooth surface that leads to surface removal via toothbrushing, eating hard foods, and even cleaning with toothpicks. As noted above, an important part of this education program is to advise patients to avoid brushing for at least 30 minutes following meals or acid challenges. This delay in brushing will allow—due to the buffering of acids from the saliva—the pH on the tooth surface to rise and remineralization to occur.8
Conservative treatment with fluoride mouthrinse and topical fluoride application should be managed by dental hygienists. Also, the use of fluoride varnish and bonding agents can protect the teeth from acid attacks, but their effects may be limited.17 Recent reports have noted that a toothpaste containing 8% arginine (an insoluble calcium compound) and calcium carbonate may be more effective for decreasing dentine permeability and for enhancing enamel remineralization than conventional high-fluoride dentifrices.18,19 This toothpaste is not available in the United States; however, an in-office paste containing arginine and calcium carbonate is currently on the market. Another important treatment option may be the use of a stannous fluoride dentifrice. Toothpastes containing stannous fluoride may provide improved resistance to tooth structure loss due to erosion and abrasion by depositing a protective layer on the tooth surface.20,21
Treatment must begin by minimizing the intrinsic or extrinsic acids that are the primary cause(s) of the lesions. Treatment for cervical hypersensitivity due to NCCLs may also be necessary. Using desensitizing dentifrices, such as those containing potassium nitrate, may be helpful. The application of an in-office arginine-calcium carbonate-sodium paste may also ameliorate hypersensitivity.22,23 Other studies have shown that the use of a self-etching adhesive, sealant, or resin-bonded flowable composite restoration can provide comprehensive and rapid control of hypersensitivity.24 More advanced lesions can usually be managed using resin-bonded composite restorations. In rare instances, a crown or porcelain veneer may be necessary.
CONCLUSION
Dental hygienists are in a unique position to identify, educate, and coordinate treatment for patients with NCCLs. Understanding the etiology of NCCLs and making a timely diagnosis will allow early and minimally invasive interventions—such as the use stannous fluoride toothpaste or arginine-calcium carbonate in-office paste, fluoride varnish, and sealants. Counseling for behavioral modifications, such as reducing the intake of acidic foods and beverages, is of upmost importance. Explaining various treatment options will help patients manage this common problem.
REFERENCES
- Bartlett DW, Shah P. A critical review of non-carious cervical (wear) lesions and the role of abfraction, erosion and abrasion. J Dent Res. 2006;85:306–312.
- Huysmans MC, Chew H, Ellwood RP. Clinical studies of dental erosion and erosive wear. Caries Res. 2011;45(Suppl 1):60–68.
- Barbour ME, Rees GD. The role of erosion, abrasion and attrition in tooth wear. J Clin Dent. 2006;17:88–93.
- Grippo JO, Simring M, Coleman TA. Abfraction, abrasion, biocorrosion, and the enigma of noncarious cervical lesions: a 20-year perspective. J Esthet Restor Dent. 2012;24:10–23.
- Lussi A, Schlueter N, Rakhmatullina E, Ganss C. Dental erosion—an overview with emphasis on chemical and histopathological aspects. Caries Res. 2011;45(Suppl 1):2–12.
- Addy M, Shellis RP. Interaction between attrition, abrasion and erosion in tooth wear. Monogr Oral Sci. 2006;20:17–31.
- von Fraunhofer JA, Rogers MM. Effects of sports drinks and other beverages on dental enamel. Gen Dent. 2005;53:28–31.
- Lussi A, Schaffner M. Progression of and risk factors for dental erosion and wedge-shaped defects over a 6-year period. Caries Res. 2000;34:182–187.
- Moore C, Addy M. Wear of dentine in vitro by toothpaste abrasives and detergents alone and combined. J Clin Periodontol. 2005;12:1242–1246.
- Harpenau L, Noble W, Kao R. Diagnosis and management of dental wear. J Calif Dent Assoc. 2011;39:225–231.
- Niemi ML, Sandholm L, Ainamo J. Frequency of gingival lesions after standardized brushing as related to stiffness of toothbrush and abrasiveness of dentifrice. J Clin Periodontol. 1984;11:254–261.
- Brandini DA, Pedrini D, Panzarini SR, Benete IM, Trevisan CL. Clinical evaluation of the association of non-carious cervical lesions, parafunctional habits and TMD diagnosis. Quintessence Int. 2012;43:255–262.
- Kuroe T, Itoh H, Caputo AA, Nakahara H. Potential for load-induced cervical stress concentration as a function of periodontal support. J Esthet Dent. 1999;11:215–222.
- Grobler S, Jenkins G, Kotze D. The effects of the composition and method of drinking of soft drinks on plaque pH. Br Dent J. 1985;158:293–296.
- Lussi A, Jaeggi, T, Zero D. The role of diet in the etiology of dental erosion. Caries Res. 2004;38:34–44.
- Farahmand F, Sabbaghian M, Ghodousi S, Seddighoraee N, Abbasi M. Gastroesophageal reflux disease and tooth erosion: a cross-sectional observational study. Gut Liver. 2013;7:278–281.
- Austin RS, Stenhagen KS, Hove LH, et al. A qualitative and quantitative investigation into the effect of fluoride formulations on enamel erosion and erosion abrasion in vitro. J Dent. 2011;39:648–655.
- Pinto SC, Bandéca MC, Pinheiro MC, et al. Preventive effect of a high fluoride toothpaste and arginine-carbonate toothpaste on dentinal tubules exposure followed by acid challenge: a dentine permeability evaluation. BMC Res Notes. 2014;7:385.
- Huang Y, Duan Y, Qian Y, et al. Remineralization efficacy of a toothpaste containing 8% arginine and calcium carbonate on enamel surface. Am J Dent. 2013;26:291–297.
- Bellamy PG1, Harris R, Date RF, et al. In situ clinical evaluation of a stabilized, stannous fluoride dentifrice. Int Dent J. 2014;64:43–50.
- Eversole SL, Saunders-Burkhardt K, Faller RV. Erosion protection comparison of stabilized SnF2, mixed fluoride active and SMFP/arginine-containing dentifrices. Int Dent J. 2014;64(Suppl 1):22–28.
- Docimo R, Monstesani L, et al. Comparing the efficacy in reducing dentin hypersensitivity of a new toothpaste containing 8.0% arginine, calcium carbonate, and 1,450 ppm fluoride to a commercial sensitive toothpaste containing 2% potassium ion: an eight-week clinical study in Rome, Italy. J Clin Dent. 2009;20:17–22.
- Sharif MO, Iram S, Brunton PA. Effectiveness of arginine-containing toothpastes in treating dentine hypersensitivity: a systematic review. J Dent. 2013;41:483–492.
- Veitz-Keenan A, Barna JA, Strober B, et al. Treatments for hypersensitive noncarious cervical lesions: a Practitioners Engaged in Applied Research and Learning (PEARL) Network randomized clinical effectiveness study. J Amer Dent Assoc. 2013;144:495–506.
From Dimensions of Dental Hygiene. October 2014;12(10):21–22,24.

