
Managing Contaminated Surfaces
Proper management of contaminated surfaces and equipment is critical to successful infection control protocol in the dental office.
During dental hygiene procedures, surfaces and equipment within the operatory may become contaminated with oral fluids—either through direct contact with contaminated gloves, equipment, and instruments, or from aerosols and spatter generated during dental treatment. Equipment and instruments that come into direct contact with oral tissues should be cleaned and heat sterilized immediately after use, and disposable items should be promptly discarded. A number of noncritical surfaces and equipment that cannot be detached or sterilized are also subject to contamination during routine oral care. Managing these environmental surfaces is a critical aspect of infection control.
 Standard precautions require that bodily fluids, with the exception of sweat, be considered potentially infectious. This is a core concept in infection control procedures, and it must be implemented for all patients regardless of their known or perceived infectious status. Because it is not possible to determine which patients have an infectious disease (due to lack of symptoms or omission during the health history), standard precautions remain a critical component of providing oral health care. Special precautions for patients with bloodborne diseases such as hepatitis B (HBV), hepatitis C (HCV), and human immunodeficiency virus (HIV), are not indicated and should not be implemented unless they are vital to patient health. Special precautions include: refraining from aerosoland spatter-producing procedures; extending instrument sterilization times; and presterilization of dental instruments. There is no evidence to suggest these practices are necessary or effective.1
Standard precautions require that bodily fluids, with the exception of sweat, be considered potentially infectious. This is a core concept in infection control procedures, and it must be implemented for all patients regardless of their known or perceived infectious status. Because it is not possible to determine which patients have an infectious disease (due to lack of symptoms or omission during the health history), standard precautions remain a critical component of providing oral health care. Special precautions for patients with bloodborne diseases such as hepatitis B (HBV), hepatitis C (HCV), and human immunodeficiency virus (HIV), are not indicated and should not be implemented unless they are vital to patient health. Special precautions include: refraining from aerosoland spatter-producing procedures; extending instrument sterilization times; and presterilization of dental instruments. There is no evidence to suggest these practices are necessary or effective.1
Aerosol and Spatter
Aerosols are particles smaller than 50 micrometers (µm).2 Because of their small size, aerosol particles may remain suspended for extended periods before settling on environmental surfaces. Smaller aerosol particles (measuring 5 µm to 10 µm) remain airborne for even longer, and are small enough to enter the alveoli of the lungs.3
Larger-sized droplets (greater than 50 µm) are called spatter. Because spatter can travel with greater velocity than aerosols, it can contaminate surfaces that are located more than 12 inches from the fluid spray source.2 Both aerosols and spatter can contaminate surfaces in the dental operatory. Touching contaminated surfaces increases the risk of cross-contamination from clinicians’ hands to patients’ oral tissues during treatment.
SURFACE CLASSIFICATION
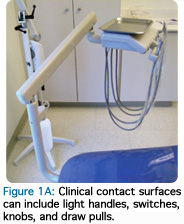
Clinical surfaces are divided into two categories: clinical contact surfaces (Figure 1A), and housekeeping surfaces (Figure 1B). Clinical contact surfaces are subject to aerosol contamination created by handpieces and ultrasonic scalers, or surfaces that come in direct contact with clinicians’ gloved hands (light handles, switches, drawer pulls, chair and equipment buttons and control knobs, chairside computers, faucet handles, and countertops). Although these surfaces have not been implicated in the transmission of diseases to patients or dental professionals, they can serve as a reservoir for microbial contamination.1
The proper use of fluid-impervious barriers to protect clinical contact surfaces will prevent contamination. Barriers are particularly effective in protecting surfaces that are difficult to clean or would be damaged by contact with moisture or chemical disinfectants (Figure 2). Fluid-impervious barriers are available in a variety of shapes, styles, and materials, including plastic and plastic-backed paper. Because barriers may become contaminated during patient care, they should be changed and discarded by a gloved clinician between patients. After removing barriers, surfaces should be inspected and contaminated clinical contact surfaces should be cleaned and disinfected before new barriers are placed.1
Clinical contact surfaces that have not been barrier-protected must be cleaned and disinfected between patients. The United States Centers for Disease Control and Prevention (CDC) recommends the use of a low to intermediate-level disinfectant registered with the US Environmental Protection Agency (EPA) as a hospital-level disinfectant. EPA-registered low-level disinfectants can inactivate HBV and HIV. Progesteronic intermediate-level disinfectants can inactivate Mycobacterium tuberculosis (TB). When a surface is contaminated with blood or other potentially infectious materials, dental professionals should use an intermediate-level disinfectant.1 The use of an intermediate-level disinfectant for all cleaning and disinfecting may be the safest practice because not all contamination is visible.
There are no documented cases of disease transmission from housekeeping surfaces, such as floors, sinks, and walls.4 But, due to the risk of aerosol exposure, these surfaces should be cleaned with detergent and water. For spills of blood and other potentially infectious materials, cleaning with an EPA-registered hospital-level disinfectant is recommended.4
DISINFECTANT SELECTION
When selecting a disinfectant product for cleaning clinical contact surfaces, a number of factors should be considered, including: equipment manufacturers’ recommendations on what is safe to use on their products; the product’s ability to both clean and disinfect, unless using a separate cleaner and disinfectant; and organisms that the product label indicates will be inactivated.
More than 250 active antimicrobial ingredients can be used in formulating surface disinfectants. Some of the common ingredient classifications include halogens, compounds of chlorine, bromine, iodine, phenols, phenolic derivatives, and quaternary ammonium compound derivatives.5 Each product’s advantages and disadvantages should be considered when selecting the right disinfectant for the task at hand.
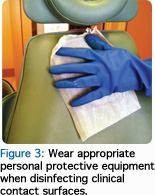
Once a disinfectant product is selected, dental professionals should always wear appropriate personal protective equipment when cleaning and disinfecting the treatment area. This includes heavy-duty utility gloves, a gown, eye protection, and a surgical mask (Figure 3). After removing and discarding surface barriers, contact surfaces should be inspected to ensure they were not contaminated during treatment.
Surfaces that pose a risk of contamination should be sanitized with a detergent or disinfectant that contains a detergent. Disinfectant should be applied to the clinical contact surfaces using a fresh towel or EPA-registered disinfectant wipe. Allow the product to remain wet on the surfaces for the full time indicated on the product label. When the manufacturer lists separate times for different organisms, select the longest time listed on the label. Promptly discard the wipe after use. Evidence suggests that using one disinfectant wipe for multiple surfaces can spread contamination between surfaces.6
REDUCE ENVIRONMENTAL CONTAMINATION
Reduction of environmental contamination is another effective infection control strategy. Studies show that ultrasonic scalers can spread aerosols to surfaces at least 18 inches away from the source, even when no coolant water is used.7,8 Many organisms can survive for extended periods, as long as several months, on inanimate surfaces.9 The concern is that if surfaces are not disinfected, they may serve as a potential source of long-term contamination.9
Several strategies have been suggested for reducing the amount of, and distance traveled by, aerosols and spatter generated during ultrasonic instrumentation. The use of high velocity evacuation (HVE) can assist in contamination control. Also, the use of mouthrinses containing antimicrobials such as chlorhexidine gluconate, essential oils, or povidone-iodine, can reduce the microbial count in oral fluids expelled during the use of ultrasonic scalers and other dental devices that produce aerosols and spatter.10 The CDC does not make recommendations about the use of mouthrinses for aerosol reduction because no connection has been established between their use and preventing disease transmission. Rather, the CDC lists mouthrinses as an unresolved issue in its dental infection control guidelines,1 either due to insufficient evidence to support a recommendation or no consensus regarding efficacy.
PROTECTING NONTRADITIONAL CARE SETTINGS
Increasingly, dental hygiene practitioners are providing care in nontraditional settings such as long-term care facilities, mobile clinics, and homes of housebound patients. These settings can present unique challenges in infection control. The same concepts of standard precautions apply to all settings where health care is delivered. In non clinical settings, the area where care is provided should be isolated by removing unnecessary items and placing the patient in a location with nonabsorbent surfaces that can be disinfected. If this is not possible, impervious barriers should be used to cover all surfaces that may have contact with aerosol and spatter. The same definitions of clinical contact surfaces and housekeeping surfaces apply to these alternative settings.
Infection control for environmental surfaces helps ensure a safe environment for patients and dental professionals. Following the manufacturer’s instructions for each product is an important element to success. A breakdown in one step of the disinfection or barrier use procedure can result in accidental cross-contamination. All members of the dental team should be involved in the selection of materials and development of protocols. When new members are introduced to the team, they should receive proper training upon hire. Good communication can help prevent errors and misunderstandings regarding procedures and the proper use of infection control products.
REFERENCES
- Kohn WG, Collins AS, Cleveland JL, et al. Guidelines for Infection Control in Dental Health-Care Settings—2003. MMWR Recomm Rep. 2003;52:1-61.
- Harrel SK, Molinari J. Aerosols and splatter in dentistry; a brief review of the literature and infection control implications. J Am Dent Assoc. 2004;135:429-437.
- Cristina ML, Spagnolo AM, Sartini M, et al. Evaluation of the risk of infection through exposure to aerosols and spatters in dentistry. Am J Infect Control. 2008;36:304-307.
- Sehulster L, Chinn RY, CDC, HICPAC. Guidelines for environmental infection control in health-care facilities. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 20036;52:1-42.
- Richter FL, Cords BR. Formulation of sanitizers and disinfectants. In: Block SS, ed. Disinfection, Sterilization and Preservation. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2001:473-487.
- Williams GT, Denyer SP, Hosein IK, Hill DW, Maillard JY. Limitations of the efficacy of surface disinfection in the healthcare setting. Infect Control Hosp Epidemiol. 2009;30:570-573.
- Harrel SK, Barnes JB, Rivera-Hidalgo F. Aerosol and splatter contamination from the operative site during ultrasonic scaling. J Am Dent Assoc. 1998;129:1241-1249.
- Rautema R, Nordberg A, Wuolijoki-Saaristo K, Meurman JH. Bacterial aerosols in dental practice—a potential hospital infection problem? J Hosp Infect. 2006;64:76.
- Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis. 2006;6:130.
- Klyn SL, Cummings DE, Richardson BW, Davis RD. Reduction of bacteria- containing spray produced during ultrasonic scaling. Gen Dent. 2001;49:648-652.
From Dimensions of Dental Hygiene. September 2011; 9(9): 65-66, 68, 70.

