Implications of Influenza
Understanding transmission, disease relationships, and prevention will help you protect yourself and your patients.
Click here for tables:
Table 1: Those at Increased Risk for Postinfluena Complications
Table 2: Representative Complication of Influenza
Table 3: Live, Attenuated Influenza Vaccine (LAIV) Compared with Inactivated Influenza
Influenza viruses have the capacity to infect a variety of animals, including birds, swine, horses, and humans. Genetic variations, which are commonly manifested by different strains, have provided influenza viruses with unique infectious and epidemiological features. As discussed in the first part of this series, these ribonucleic acid (RNA) viruses have caused numerous human epidemics and pandemics, along with resultant substantial mortality.1 The most severe recorded outbreak was the Spanish flu of 1918-1919. During this pandemic, more than 21 million people died worldwide, with over 500,000 deaths in the United States.
TRANSMITTING THE INFLUENZA VIRUS
The flu is spread from infected persons by transfer of microbial-laden secretions in respiratory droplets. Depending on the viral load within the droplets, infectious concentrations of influenza viruses can survive evaporation of the respiratory particles and remain suspended in the air for extended intervals. Viral passage may therefore occur to susceptible persons who are within the same room, but not in close proximity to infected hosts. Maximum communicability between people usually occurs 1-2 days before onset of symptoms through 4-5 days after onset of clinical illness. Children can remain infectious for 1o days or more.
The relatively sudden onset of disease symptoms plays a major role in the efficiency of viral transmission. Since high concentrations of virus are present in respiratory secretions from those exhibiting influenza symptoms, small particle aerosols created by sneezing, coughing, or even talking, can disperse sufficient virulent material from a single infected host to numerous susceptible people.2 Clinical manifestations of uncomplicated influenza then develop suddenly after 1-5 days (usually 2), with the most prominent physical symptom being a rapidly developing fever (100° to 104° F) within 12 hours of symptoms onset. Type A influenza viruses are relatively stable under a variety of environmental conditions, especially low temperature and humidity. This characteristic facilitates transmission of these pathogens to the exclusion of many other airborne pathogens.
While the above description suggests that influenza viruses survive and may be transmitted from contaminated fomites (inanimate objects that are not harmful but can harbor pathogenic microorganisms), such as bed linens, table tops, counters, and other objects, little available information supports this possibility. The potential of influenza infection via fomites remains poorly understood. Despite a lack of evidence for this potential transmission modality, health care-ssociated, ie, nosocomial, influenza continues to be a serious problem. Hospitals in particular house many patients who have an increased risk for life-threatening post-influenza illnesses. As a consequence, standard aseptic procedures should be followed when caring for influenza patients in all health care facilities. These include routine hand hygiene and wearing appropriate personal protective equipment (especially masks). Dental professionals have a long history of incorporating these types of practices in their universal (now “standard”) infection control precautions. Thus, the major occupational influenza risk for dental health care providers is from exposure to airborne, viralladen droplets.
DISEASE COMPLICATIONS
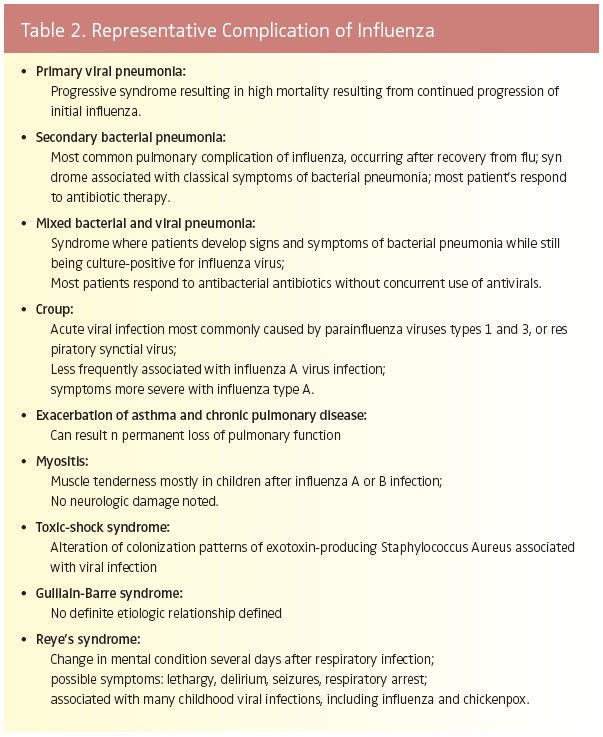
Pulmonary sequelae to influenza often occur in high risk patients, although non-pulmonary complications may also develop. The most common condition developed is pneumonia. The etiology of influenza-induced pneumonia may be viral, bacterial, or mixed viral-bacterial, with secondary bacterial pneumonia being the most frequently reported.3
People at increased risk for postinfluenza illness include sick patients who are elderly or very young, have chronic pulmonary disease, are pregnant, or are immunocompromised from other conditions (Table 1).4 Those who develop secondary bacterial pneu- monia appear to initially recover from classical influenza for intervals ranging from a few days to 2 weeks. This quiescent period is followed by onset of fever, a chronic cough, sputum production, and other signs associated with respiratory bacterial infection. Culture and gram staining of patients’ sputum commonly reveals the presence of Streptococcus pneumoniae, Haemophilus influenzae, or Staphylococcus aureus as the etiology. Antibiotic regimens and respiratory supportive therapy are necessary for successful treatment in these instances. Even with these disease interventions, many strains of S. pneumoniae and S. aureus are resistant to multiple antimicrobial agents, and thus, early diagnosis of bacterial infection is very important in order to successfully treat patients.
An uncommon but more catastrophic sequela is primary viral pneumonia. This lifethreatening condition was first documented during the 1957-1958 Asian flu pandemic and continues to occur in high-risk influenza patients with cardiovascular or pulmonary diseases. Investigations of the 1918-1919 Spanish flu also indicate that this progressive respiratory disease caused many influenza deaths in young adults. Unlike previous outbreaks, death rates among healthy young adults during the Spanish flu pandemic were higher than those recorded for the very young and the elderly. The virulence of the etiologic viral strain was noted as a prime factor responsible for the exceptionally high fatality incidence. 5
A variety of serious, non-pulmonary sequelae can also occur after primary influenza illness. In particular, a number of chronicmedical disorders, such as asthma and congestive heart failure, can also be exacerbated when the person develops influenza.3,6
CONTROLLING INFLUENZA
The primary approach for reducing the morbidity and mortality of influenza virus infection involves immunoprophylaxis through vaccination. Two influenza vaccines are currently approved for use, an inactivated (killed virus) preparation and a live, attenuated influenza vaccine (LAIV). These are manufactured annually as trivalent vaccines, directed against three influenza virus strains (two subtypes of influenza Aviruses and one type B virus). The choices for inclusion are the most probable, frequently occurring strains for the upcoming flu season, as determined by reference laboratories representing the World Health Organization and the Centers for Disease Control and Prevention. Improvements in global influenza virus surveillance over the years have resulted in better antigenic matches between vaccine and circulating viral strains. Each year one or more of the virus strains may be changed based on global surveillance for influenza viruses and the emergence/spread of new strains.4 Although both humoral and cellular immune responses develop, vaccination primarily stimulates protective antibodies against influenza hemagglutinin (HA) and neuraminidase (NA) antigens.
Inactivated influenza vaccines remain the major public health control measure and have been in use since 1947. Although previously approved preparations used whole killed viruses, sub-virion influenza vaccines have been produced since the 1970s to reduce potential toxicity from non-immunogenic viral components. Only disrupted, or split-virus vaccines, are currently available as inactivated influenza preparations in the United States. Following intramuscular injection, inactivated flu vaccines stimulate rapid antibody synthesis within 2 weeks in healthy young adults with peak serum immunoglobulin levels noted within 4 to 6 weeks postvaccination.7,8
A new LAIV was licensed for use in 2003. This alternative vaccination approach was pursued because it provides the benefits of prolonged viral replication within the host (thereby offering more immunogenic stimulation) and is easier to administer via a nasal spray rather than injection. This preparation, available as FluMist, contains the same influenza virus strains as the inactivated vaccine. It is approved for administration to healthy children and adults 5 to 49 years old, including those who are household contacts of people at high risk for postinfluenza complications. The weakened viruses in the LAIV are attenuated to be cold-adapted and temperature sensitive, which means the microbes can grow in the nose and throat, but not in the lower respiratory tract where the temperature is higher.9 As a result the altered viruses can be well tolerated by the vaccinated host in addition to being highly efficacious at stimulating both serum IgG and secretory IgA antibodies for immunity.
With regard to public health protocols for influenza vaccination, administration via intramuscular injection or nasal spray is recommended during September and October just prior to onset of the annual flu season. This time frame allows for protective antibody concentrations to develop before the arrival of peak influenza months. The only contraindication to vaccination is an allergy to eggs, the medium used for growth of influenza viruses.
Antiviral drugs can also be used to combat influenza. Amantadine and rimantadine are the most widely used agents in preventing clinical illness from influenza A virus infection. However, they are not indicated for chemicoprophylaxis against influenza B viruses.10,11 While antiviral chemotherapy is not a substitute for vaccination, these drugs serve as adjuncts to vaccines by preventing clinical influenza in approximately 75% to 90% of young adults studied.12 When antiinfluenza agents are used to treat patients with the disease, early intervention with the antivirals increases the outlook for patient recovery and eradication of the viruses.
Dr. Molinari is available for questions through the Ask the Expert section of Dimensions’ website at www.dimensionsofdentalhygiene.com.

REFERENCES
- Molinari JA. Fighting the flu. Dimensions of Dental Hygiene. 2005;3(1):22-26.
- Treanor JJ. Influenza virus. In: G Mandell, JE Bennett, R Dolin, eds. Principles and Practice of Infectious Disease. 5th ed. Philadelphia: Churchill Livingstone; 2004:1823-1849.
- Cate TR. Clinical manifestations and consequences of influenza. Am J Med. 1987;82:15-19.
- Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB, Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP). Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2004;53(RR-6):1-40.
- Cunha BA. Influenza: historical aspects of epidemics and pandemics. Infect Dis Clin North Am. 2004;18:141-155.
- Ljungman P, Andersson J, Aschan J, et al. Influenza A in immunocompromised patients. Clin Infect Dis. 1993;17:244-247.
- Brokstad KA, Cox RJ, Olofsson J, Jonsson R, Haaheim LR. Parenteral influenza vaccination induces a rapid systemic and local immune response. J Infect Dis. 1995;171:198-203.
- Gross PA, Russo C, Dran S, Cataruozolo P, Munk G, Lancey SC. Time to earliest peak serum antibody response to influenza vaccine in the elderly. Clin Diagn Lab Immunol. 1997;4:491-492.
- Centers for Disease Control and Prevention. Questions & Answers: The Nasal-Spray Flu Vaccine (Live Attenuated Influenza Vaccine). Available at: www.cdc.gov/flu/about/qa/nasalspray.htm. Accessed January 13, 2005.
- Demicheli V, Jefferson T, Rivetti D, Deeks J. Prevention and early treatment of influenza in healthy adults. Vaccine. 2000;18:957-1030.
- Tominack RL, Hayden FG. Rimantatine hydrochloride and amantadine hydrochloride use in influenza A virus infections. Infect Dis Clin North Am. 1987;1:459-478.
- Dolin R, Reichman RC, Madore HP, Maynard R, Linton PN, Webber-Jones J. A controlled trial of amantadine and rimantadine in the prophylaxis of influenza A infection. N Engl J Med. 1982;307:580-584.
From Dimensions of Dental Hygiene. February 2005;3(2):22-24.