
Implant Maintenance
Technique and tools for effective debridement of artificial anatomy.
This course was published in the January 2011 issue and expires January 2014. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Discuss the anatomy of the bone level fixture and adjacent tissues.
- Describe the role of plaque-induced inflammation in peri-implant tissues.
- Explain the presented classification system for implant maintenance.
- Identify the suggested armamentarium for implant maintenance.
The accumulation of plaque on the natural dentition initially occurs supragingivally and over time extends subgingivally, resulting in the formation of gingivitis.1 Professional maintenance care reduces the potential for ensuing attachment loss. The goal is to remove plaque and calculus from the clinical crown surface as well as the surface of tooth structure adjacent to the sulcus or pocket. These basic premises of periodontal therapy are integral to implant therapy because the bacteria associated with periodontal diseases are the same as those associated with peri-implantitis.2
The recommended instrumentation technique to debride the artificial anatomy, however, is quite different from periodontal instrumentation. Instruments used to remove plaque and calculus are often made of plastic or titanium. The purpose of using these plastic or titanium materials is to prevent or reduce scratching of the abutment or the implant surface, which may increase the potential for increased plaque retention.
A probing depth greater than 3 mm in a periodontal pocket may hinder the patient’s ability to adequately clean the area. The literature indicates that probing depths greater than 4 mm around the natural dentition make professional root debridement more difficult.3 In addition to debridement, a smooth root surface is one of the desired outcomes of treatment. In the case of dental implants, however, there is no root surface to debride. If probing depths exceed 3 mm, what are we as dental professionals debriding? To answer this question, the morphology of the restored bone level implant fixture must be examined.
ANATOMY
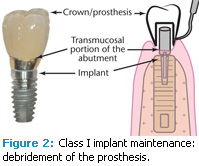
The generic bone level restored implant consists of three components: the crown, the abutment, and the implant fixture (Figure 1). The abutment is the connection between the crown and the dental implant. It consists of three portions: the prepared portion on which the crown is cemented, the transmucosal portion adjacent to the sulcus of the implant, and the most apical portion that engages the implant and is held in place by a retaining screw.
In an ideal circumstance, the restored implant fixture itself is not exposed to the oral cavity, but rather, integrated to the surrounding bone. A 3 mm to 4 mm vertical dimension of peri-implant mucosa exists coronal to the bone. The apical portion of the peri-implant mucosa measures 1 mm to 2 mm occluso-apically and consists of dense connective tissue.
 Coronal to the connective tissue is a zone of soft tissue that attaches to the implant via a junctional epithelium. Coronal to the epithelial attachment is a soft tissue cuff that comprises the sulcus, which is adjacent to the transmucosal portion of the abutment and the crown. The base of that sulcus is apical to the interface between the implant and the abutment. For restored bone level implants, the interface between the implant and the abutment is known as the microgap. In other words, for restored bone level implants, the epithelial attachment is not on the abutment. The epithelial attachment is apical to the microgap and is located on the implant fixture.4
Coronal to the connective tissue is a zone of soft tissue that attaches to the implant via a junctional epithelium. Coronal to the epithelial attachment is a soft tissue cuff that comprises the sulcus, which is adjacent to the transmucosal portion of the abutment and the crown. The base of that sulcus is apical to the interface between the implant and the abutment. For restored bone level implants, the interface between the implant and the abutment is known as the microgap. In other words, for restored bone level implants, the epithelial attachment is not on the abutment. The epithelial attachment is apical to the microgap and is located on the implant fixture.4
The suprabony soft tissues form the “biological width” for the implant.5 These tissues are analogous to the biological width associated with the natural dentition. Both have an epithelial component; the main difference is in the nature of the connective tissue component. It is relatively avascular and does not attach to the implant via a periodontal ligament.
PLAQUE REMOVAL
Despite the fact that there is reduced vascularity associated with peri-implant tissues, they are still susceptible to plaque inducedinflammation.6 The unattached portion of suprabony soft tissues provides an area for subgingival plaque accumulation similar to a sulcus or pocket around a natural tooth. The rationale for good plaque control measures and implant maintenance is to remove implant-associated biofilm. The bacteria, if left undisturbed, may result in a peri-implant mucositis, which is similar to gingivitis.7
Plaque-induced inflammation may also result in bone loss causing partial de-integration of the implant from the adjacent bone.8 If the implant is not mobile and is still functioning, this condition is referred to as peri-implantitis. If the bone loss associated with peri-implantitis is progressive, the implant may become mobile. A failed implant is defined as a mobile fixture.
Clearly, removal of plaque around restored implants is an important objective to prevent peri-implant mucositis and reduce the risk of peri-implantitis. To facilitate a practical approach to maintenance therapy, the relationship between the implant and various conditions of the hard and soft tissues adjacent to the restored implant fixture should be classified.
Classification System
Class I implant maintenance. When health is present in an ideal relationship between the peri-implant tissues and the restored implant, the therapist does not actually clean any portion of the implant fixture. Rather, the therapist is most likely just debriding the crown/prosthesis. In this case, maintenance could be classified as Class I implant maintenance—debridement of the prosthesis (Figure 2).
Class II implant maintenance. When probing depths are 4 mm or greater or some moderate peri-implant soft tissue recession has occurred, the therapist may have access to debride the transmucosal portion of the abutment. In this case, maintenance could be classified as Class II implant maintenance— debridement of the prosthesis and the transmucosal portion of the abutment (Figure 3). The height of the abutment may vary because of varying soft tissue heights or countersinking of the implant in the esthetic zone. This will result in probing depths greater than 3 mm but does not represent breakdown of the peri-implant tissues.
Class III implant maintenance. When significant recession or pocket formation and concomitant bone expose the actual implant, the therapist is compelled to debride the exposed portion of the implant fixture. This is not an ideal situation because the implant has lost some bone support, which is analogous to attachment loss on a natural tooth. In this clinical scenario, the exposed coronal aspect of the implant may have a polished metal collar or a roughened surface that can be more plaque retentive than a polished metal collar. Worse yet, exposure may include threads that may confound debridement. In this clinical circumstance involving exposure of the implant, maintenance could be classified as Class III implant maintenance—debridement of the prosthesis, the transmucosal portion of the abutment, and the exposed implant fixture (Figure 4).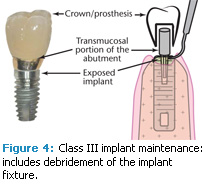
IMPLANT INSTRUMENTATION
Commercially available products for implant instrumentation are typically manual plastic scalers, manual titanium scalers, and ultrasonic scalers covered with plastic or carbon composite tips.9 These instruments are designed to reduce or eliminate the possibility of scratching the implant surface. Air abrasives are also used in implant maintenance.10
Scratching of titanium surfaces by conventional metal instrumentation was demonstrated in vitro by Fox et al.11 These investigators compared stainless steel and titanium alloy curets to plastic instruments when scaling a new smooth cylindrical titanium abutment. All three were compared to a control: an untreated smooth titanium abutment. The abutments were subsequently examined under a microscope. Both types of metal curets produced surface roughness on the abutments. The titanium curets produced less roughness than the stainless steel curets. The plastic curets produced a surface similar to the control (an untreated abutment). In other words, plastic curets did not scratch the titanium abutment.
In a subsequent study, Rapley and colleagues also compared metal curets to plastic curets on titanium abutment surfaces in an in vitro study. Their results were similar to Fox et al.12 As a result of these investigations, the general consensus was that plastic curets were the instruments of choice to use during implant maintenance therapy. The rationale for not using conventional instrumentation around implants is that scratching the smooth surface of the abutment or implant will make it more plaque retentive.
An in vivo investigation by Quirynen and colleagues demonstrated increased plaque retention when abutments with rough surfaces were present compared to smooth abutments.13 Additional research on the potential outcome of scratching the abutment and its clinical relevance is needed. A recent animal study indicates that a rough surface titanium abutment failed to influence plaque-associated peri-implant mucositis when compared to a smooth abutment.14
In the Class III maintenance scenario (exposure of the implant) newer implant designs present an interesting clinical situation. The concept of preventing scratching of the actual implant surface may be regarded as contradictory in light of the fact that a number of commonly used implant systems have eliminated the polished collar associated with the most coronal aspect of the implant. These implants have a roughened surface extending to the most coronal portion of the implant fixture. In the case of implants with a completely roughened surface, recent evidence indicates that metal curets may actually reduce implant surface roughness, making them less plaque retentive.15
Another concern is that a stainless steel instrument will contaminate the implant surface. But if the fixture is exposed, it is already contaminated with plaque and possibly calculus. Metal contamination from a metal ultrasonic tip is probably less detrimental to the surrounding peri-implant tissues than bacterial contamination. Clearly, changes in implant design in addition to metal’s potential to more effectively remove contamination of exposed implants should encourage a reassessment of the instrument armamentarium used during implant maintenance.
NEW ARMAMENTARIUM
For a Class I clinical scenario, the armamentarium may include a plastic curet. As an alternative, the same instruments used for the natural dentition can also be incorporated. Consider this clinical scenario: a therapist debrides calculus on the lingual aspect from a porcelain fused- to-metal mandibular anterior fixed prosthesis associated with natural dentition. No attachment loss is noted around the involved dentition. Would plastic scalers be able to remove the calculus effectively? Would conventional ultrasonic instrumentation damage the prosthesis? If the answer to both of these questions is no, then the same instrumentation can be used to debride plaque and calculus from porcelain-fused-to-metal mandibular anterior fixed prosthesis associated with generic bone level implants.
For a Class II clinical scenario, the armamentarium may include conventional metal and ultrasonic instruments to debride the prosthesis. Plastic scalers and/or plastic tipped ultrasonic instrumentation could be used to debride any exposed supragingival and subgingival portions of the abutment so not to roughen it.
For a Class III clinical scenario, the armamentarium may include conventional metal and ultrasonic instruments to debride the prosthesis. Plastic scalers and/or plastictipped ultrasonic instrumentation could be used to debride any exposed supragingival and subgingival portions of the abutment.
Air abrasive devices may be considered for use in all three classifications. The in vitro efficacy of debridement with air polishing has been demonstrated.16 However, alteration of the abutment or implant surface can occur with increased time of exposure. Caution must be used with this device because significant trauma can occur to the surrounding soft tissue.17 Moreover, improper use of an air abrasive device can result in tissue emphysema.
IS STANDARDIZATION NECESSARY?
The need for a standardization of instruments used to debride the exposed implant is debatable. While an implant with a polished metal collar would not be scratched with a plastic scaler, an implant with a manufactured roughened surface or with exposed threads would be difficult to debride with plastic. In addition to the possible ineffectiveness of the plastic scaler, the manufactured roughened surface or the threads on the implant might abrade the plastic scaler. As a result, small plastic fragments may be left residing on the roughened implant surface.18 These same surface types could also abrade a plastic tip covering an ultrasonic scaler. Moreover, exposed threads could abrade that plastic tip, leaving plastic fragments in the subgingival area (Figure 5).
There is evidence that pockets around the natural dentition may serve as a reservoir for pathogenic bacteria that allows other areas of the periodontium to become infected.19 Natural teeth may also serve as bacterial reservoirs for infection of periimplant issues.20 Considering this possibility, thorough debridement of the natural dentition may be another critical componentof the maintenance of restored implants in a partially edentulous patient.
Removal of the biofilm associated with restored implant fixtures is the goal of implant maintenance therapy but the instruments employed to do this should be chosen relative to the condition of the periimplant tissues adjacent to the restored implant. Conventional metal instrumentation, both manual and ultrasonic, may be used when cleaning the implant-supported prosthesis or when the implant, particularly a roughened surface implant, is exposed. Air abrasives may also be used, although with caution. A knowledge of implant anatomy is key in order to debride these surfaces safely and effectively.
REFERENCES
- Löe H, Theilade E, Wright WH, Jensen SB. Experimental gingivitis in man. A longitudinal clinical and bacteriological investigation. J Periodontol. 1965;36:177-187.
- Mombelli A, van Oosten MA, Schurch E Jr, Land NP. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol Immunol. 1987;2:145-151.
- Stambaugh RV, Dragoo M, Smith DM, Carasali L. The limits of subgingival scaling. Int J Periodontics Restorative Dent. 1981;1:30-41.
- Berglundh T, Berglundh T, Lindhe J, et al. The soft tissue barrier at implants and teeth. Clin Oral Implants Res. 1991;2:81-90.
- Berglundh T, Lindhe J. Dimension of the periimplant mucosa. Biological width revisited. J Clin Periodontol. 1996;23:971-973.
- Pontoriero R, Tonelli MP, Carnevale G, Mombelli A, Nyman SR, Lang NP. Experimentally induced periimplant mucositis. A clinical study in humans. Clin Oral Implants Res. 1994;5:254-259.
- Zitzmann NU, Berglundh T, Marinello C, et al. Experimental peri-implant mucositis in man. J Clin Periodontol. 2001;28:517-523.
- Tonetti MS. Rsik factors for osseodisintegration. Periodontol 2000. 1998;17:55-62.
- Pattison AM. Ongoing innovation. Dimensions of Dental Hygiene. 2006;4(11):18-20.
- Dennison DK, Huerzeler MB, Quinones C, Caffesse RG. Contaminated implant surfaces: an in vitro comparison of implant surface coating and treatment modalities for decontamination. J Periodontol. 1994;65:942-948.
- Fox SC, Moriarty ID, Kusy RP. The effects of scaling a titanium implant surface with metal and plastic instruments: an in vitro study. J Periodontol. 1990;61:485-490.
- Rapley JW, Swan RH, Hallmon WW, Mills MP. The surface characteristics produced by various oral hygiene instruments and materials on titanium implant abutments. Int J Oral Maxillofac Implants. 1990;5:47-52.
- Zitzmann NU, Berglundh T, Marinello C, et al. Experimental peri-implant mucositis in man. J Clin Periodontol. 2001;28:517-523.
- Duarte PM, Reis AF, de Freitas PM, Ota-Tsuzuki C. Bacterial adhesion on smooth and rough titanium surfaces after treatment with different instruments. J Periodontol. 2009;80:1824-1832.
- Augthun M, Tinschert J, Huber A. In vitro studies on the effect of cleaning methods on different implant surfaces. J Periodontol. 1998;69:857-864.
- Chairay JP, Boulekbache H, Jean A, Soyer A, Bouchard P. Scanning electron microscopic evaluation of the effects of an air-abrasive system on dental implants: a comparative in vitro study between machined and plasma-sprayed titanium surfaces. J Periodontol. 1997;68:1215-1222.
- Kozlovsky A, Artzi Z, Nemcovsky CE, Hirshberg A. Effect of air-polishing devices on the gingiva; histologic study in the canine. J Clin Periodontol. 2005;32:329-334.
- Ramaglia L, di Lauro AE, Morgese F, Squillace A. Profilometric and standard error of the mean analysis of rough implant surfaces treated with different instrumentations. Implant Dent. 2006;15:77-82.
- Quirynen M, Listgarten MA. Distribution of bacterial morphotypes around natural teeth and titanium implants ad modum Brånemark. Clin Oral Implants Res. 1990;1:8-12.
- Levy RM, Giannobile WV, Feres M, Haffajee AD, Smith C, Socransky SS. The effect of apically repositioned flap surgery on clinical parameters and the composition of the subgingival microbiota: 12-month data. Int J Periodontics Restorative Dent. 2002;22:209-219.
From Dimensions of Dental Hygiene. January 2011; 9(1): 58-61.



