Face the Threat
Antibiotic resistance is a global health problem that can be addressed through education, effective infection control protocols, and prudent prescription practices.
This course was published in the April 2014 issue and expires April 30, 2017. The author has no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Define antimicrobial resistance (AMR).
- Identify the populations most vulnerable to AMR.
- Explain how AMR develops.
- Discuss the United States Centers for Disease Control and Prevention’s “Antibiotic Resistance Threat Report.”
Until the late 1920s, infections generally went untreated or were treated with home remedies. Bacteriologist Alexander Fleming inadvertently discovered penicillin in his laboratory when he observed a fungus killing bacterial cells in a petri dish. The mass production of penicillin began in the 1940s during World War II, and the need was great. Penicillin was frequently used in the military to treat battlefield wounds, but the drug did not become widely available to consumers until 1945.1
Purified penicillin demonstrated effective antibacterial activity against a wide range of bacteria and had low toxicity in humans, which made it a popular treatment modality. During the 1980s, drug companies shifted their focus to producing antiviral drugs, so the development of new antibiotics decreased substantially. Many medical professionals believed that bacterial infections were a thing of the past, so the urgency to create new antibiotics decreased. Loose prescription protocols coupled with patients requesting antibiotics unnecessarily led to the development of antimicrobial resistance (AMR).2 Since the early 1990s, AMR has become increasingly problematic.
THE PROBLEM
AMR is a process in which microorganisms develop a resistance to medications designed to destroy them. Standard treatments for diseases are no longer effective when microorganisms develop resistance, which can lead to prolonged illness and even death.
Virtually all microorganisms, including bacteria, fungi, viruses, and parasites, have developed some sort of resistance to antibiotics.3 When microorganisms are exposed to antimicrobial medications, they naturally develop traits that lead to resistance. Resistant traits are passed along during the replication process; with each cell division, resistance is spread. Several factors impact AMR, such as poor infection control practices, insufficient diagnosis, alternative treatments to antibiotics, inappropriate use of antimicrobial medicines, and weak surveillance and monitoring systems.3
AMR is a serious health threat. More than 2 million people in the United States acquire antibiotic-resistant infections annually, with 23,000 deaths directly attributed to these infections.4,5 Since 1940, about 150 types of antibiotics have been developed, but the creation of new drugs is limited by several factors.6 Many pharmaceutical companies have stopped funding research on new antibiotic drugs due to lack of profitability and the US Food and Drug Administration’s (FDA) complicated approval process.7
AMR is a global problem affecting every country in the world. Antibiotic-resistant microorganisms have been called “nightmare bacteria” because they pose a significant health threat worldwide.4 Antibiotic-resistant microorganisms can spread rapidly across the globe in today’s mobile society. In 2011, the World Health Organization (WHO) estimated 630,000 cases of multidrug resistant tuberculosis (MDR-TB) were identified in 84 countries (out of 12 million cases of TB). Approximately 3.7% of all new cases of TB are multi-drug resistant.
AMR is most deadly in under-resourced countries in Asia and Africa. The main AMR threats include TB, malaria, severe acute respiratory syndrome (SARS), methicillin-resistant Staphylococcus aureas (MRSA), and many Gram-negative bacterial infections, such as Escheria coli. MDR-TB is most prevalent in Asia, with 10% of cases noted as extremely resistant. No data exist on MDR-TB cases in sub-saharan Africa. Resistance to artemisinin (a drug used to treat malaria) has become a major concern in Thailand. In Asia, SARS-related deaths in children younger than 5 top 1.4 million every year, so the emergence of resistance to first-line treatments is of grave concern. Many diarrheal diseases are becoming resistant to ?-lactams (mainly carbapenems) in India and southern Europe. The prevalence of MRSA in Pakistan and India is growing.8
The use of antibiotics is a major factor leading to Clostridium difficile infection, a severe and sometimes life-threatening diarrheal disease that can lead to serious inflammation of the colon. Approximately 250,000 people are hospitalized each year in the US with C. difficile, causing 14,000 deaths. When antibiotics are consumed, they wipe out both the good and bad bacteria from the gastrointestinal tract for several weeks, leaving patients at risk for acquiring other more serious infections, such as C. difficile, during this time.
Antibiotics are the most commonly prescribed drugs in the world. Many antibiotic-resistant infections are preventable because nearly 50% of all prescribed antibiotics are taken unnecessarily.4 A study by Hecker et al9. reported that 30% of antibiotics were unnecessarily prescribed in hospitalized patients. A study by Macfarlane et al10 found that 87% of patients believed antibiotics would help their respiratory symptoms, with one-fifth specifically asking their physician to prescribe antibiotics.
Antibiotics are often used in animals within the food supply to promote growth and prevent disease, even though this therapy is not necessary. Recent guidance from the FDA includes a plan to phase out this practice.4 The impact of antibiotic use in animals and its effects on humans is not clearly known, but additional, unnecessary antibiotic use is a major risk factor contributing to AMR.4 Residual antibiotics used in food-producing animals may affect humans because resistant pathogens can be transferred through consumption of the animal and its byproducts. The resistant genes are then transferred from the animal to the human bacterial flora.3
VULNERABLE POPULATIONS
Vulnerable populations are at increased risk of complications and/or death due to AMR infections. A recent study by Wendt et al11 showed that 71% of C. difficile cases in US children were community based, meaning unassociated with a hospital stay; in comparison, two-thirds of adult cases of C. difficile infections are hospital acquired. The study also found that in 73% of the community-based cases, children were prescribed antibiotics for a range of issues—including ear, sinus, or respiratory infections—in the 12 weeks prior to the C. difficile infection.11
Deaths due to AMR infections in under-resourced countries, such as Asia and Africa, are alarming. In southern Asia, an estimated 96,000 deaths annually are attributed to AMR. Poor infection control practices during and after childbirth in under-resourced countries are also related to AMR. Many of these infections cannot be treated with traditional first-line antibiotics, so more expensive treatments must be employed. Unfortunately, under-resourced countries do not have these alternate antibiotics at their disposal, resulting in increased mortality rates.8
Older adults are another vulnerable group at risk of contracting AMR infections. A recent study by Bonomo12 found that antibiotics account for nearly 40% of all prescribed medications in long-term care facilities (LTCFs). The frequent transporting of patients to and from acute care facilities, lapses in hand washing practices among staff, excessive use of antibiotics, and the immunocompromised status of older adults make LTCFs ripe for AMR infections.12 Striking a balance among effective treatments in this population is difficult for health care workers. Bonomo recommends clinicians be “ecologically responsible” when prescribing antibiotics. Guidelines established by the Society for Healthcare Epidemiology of America to reduce AMR recommend antibiotic restrictions, nontreatment of asymptomatic bacterial infections, minimal use of topical antibiotics, hand washing, and continuing education for clinicians and patients.12
Immunocompromised patients are at high risk of complications when effective antibiotics are unavailable. Patients undergoing chemotherapy, dialysis, organ transplantation, and surgery have difficulty fighting infections, so the addition of antibiotic-resistant bacteria further inhibits their ability to heal. These patients rely on antibiotics to fight secondary infections. Patients in the hospital are more susceptible to infections due to weakened immune systems, so most cases of antibiotic-resistant infections occur in the hospital setting, known as hospital- or health care-acquired infections.4,13
The financial impact of AMR infections is staggering, with estimates of $20 billion in additional health care costs and $35 billion in lost productivity.4 People with antibiotic-resistant infections have prolonged hospital stays, additional physician visits, more expensive treatments, and greater disability and deaths than those without these types of infections.4 More than 8 million additional hospital days are attributed to AMR infections.4 Traditional first- and second-line antibiotic treatment measures do not work in patients with resistant infections. Health care providers end up using more expensive, less effective, and more toxic antibiotics.
HOW ANTIBIOTIC RESISTANCE OCCURS
AMR occurs through an evolutionary process. Organisms have a variety of individual traits—for example, the ability to withstand an attack from drugs like antibiotics. When antibiotics are taken, the drug kills the bacteria but also leaves behind the “resistant” traits. These now-resistant bacteria multiply significantly (almost a millionfold per day) and become the predominant microorganisms.

Drug resistance is a natural phenomenon that occurs with all types of microorganisms. It is imperative that modern medicine keep ahead of the resistance by continually developing new drugs. Resistant microorganisms render antibiotics useless by interfering with their mechanism of action, such as cell wall alteration, protein binding, and ribosomal binding. Gene acquisition happens in three ways: spontaneous DNA mutation; gene transformation, in which one bacterium takes up DNA from another; and DNA plasmids that move from one bacteria to another. A single plasmid can provide a variety of resistant strains.2
Antibiotic resistance occurs through a chain of events (Figure 1).4 First, bacteria are present and a few of them are resistant to drugs. Second, antibiotics are given to treat an illness. Antibiotics kill both the good and harmful bacteria. Third, the remaining drug-resistant bacteria take over and grow at an incredible rate. Fourth, the antibiotic-resistant bacteria pass along their resistance to other bacteria during replication, creating further problems.4 Bacteria will continue to evolve and develop antibiotic resistance, so an aggressive plan of action to prevent further spread of resistance is needed.4
CENTERS FOR DISEASE CONTROL AND PREVENTION REPORT
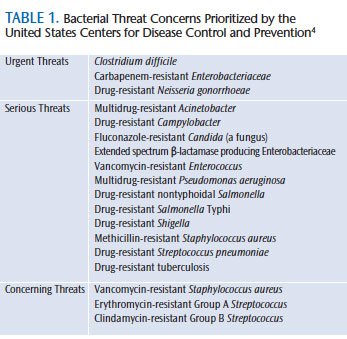
The US Centers for Disease Control and Prevention (CDC), an agency within the US Department of Health and Human Services, is the nation’s leading health organization designed to protect the public from health threats. It conducts timely, cutting-edge research on emerging diseases. The CDC created a media buzz when it released its comprehensive 114-page “Antibiotic Resistance Threats in the United States, 2013 Report” on September 16, 2013.4 The primary purpose of the report—the first of its kind—was to increase awareness about the complex problem of antibiotic resistance and to encourage action to address the issue. Written for a diverse audience, the report also serves as a valuable reference. It prioritizes bacterial threats into three categories: urgent, serious, and concerning (Table 1).4 Seven factors associated with antibiotic-resistant infections were assessed: clinical impact, economic impact, incidence, 10-year projection of incidence, transmissibility, availability of antibiotics, and barriers to prevention.4
Bacterial threats in the “urgent” category are not currently widespread—but have the potential to be—and require immediate identification and management to limit further transmission. Bacteria in this category include: C. difficile, Carbapenem-resistant Enterobacteriaceae, and drug-resistant Neisseria gonorrhoeae.
Bacterial threats in the “serious” category are not considered urgent, but will worsen without further prevention efforts. Bacteria in this category range from multidrug-resistant Acinetobacter to drug-resistant TB.
Bacteria in the “concerning” category are at low risk of antibiotic resistance, but require monitoring. Vancomycin-resistant Staphylococcus aureus, erythromycin-resistant group A Streptococcus, and clindamycin-resistant group B Streptococcus encompass this category. Gram-negative bacteria are the most concerning because they are becoming resistant to nearly all antibiotics. Some Gram-positive bacteria are also showing resistance, but at a slower rate.
Antibiotics are used to fight disease but they can exert harmful side effects, such as allergic reactions, severe diarrhea, and negative interactions with other medications. Allergic reactions constitute 140,000 emergency department visits per year in the US, with 79% (four out of five) due to adverse antibiotic reactions. Approximately one in 5,000 exposures to a parenteral dose of a penicillin or cephalosporin antibiotic causes anaphylactic shock. Antibiotics, when taken unnecessarily, may result in unwanted side effects, lack of benefits, and can lead to antibiotic resistance. The risk of developing an antibiotic-resistant infection increases each time antibiotics are taken unnecessarily.4
There are gaps in knowledge about antibiotic resistance. At the local, state, and federal levels, there is limited capacity to detect and respond to antibiotic resistance threats. There is also no global surveillance system in place to address this risk. National data on antibiotic use are not collected systematically. Programs for improving prescribing practices are not regularly utilized but could have a positive impact on the pattern. Technological advances, such as advanced molecular detection, are not routinely used in the US to detect antibiotic resistance.4
Current CDC initiatives to fight antibiotic resistance include four core actions: preventing infections and the spread of disease, tracking, improving antibiotic prescribing/stewardship, and developing new drugs and diagnostic tests. The first core action includes preventing infections and the spread of resistance. Activities such as immunization, safe food handling, hand washing, and using antibiotics only when needed are the first lines of defense. Avoiding infections in the first place reduces the need for antibiotics and, thus, the spread of resistant bacteria.
Core action two includes tracking data and risk factors associated with resistant infections, which allows experts to continue to develop prevention strategies and provide guidance to the health care community.
Core action three—improving antibiotic prescribing/stewardship—probably has the greatest impact on antibiotic resistance. Changing prescribing practices will greatly impede the spread of antibiotic resistance. A commitment to prescribe antibiotics only when needed is described as “antibiotic stewardship.” If all health care providers can become “good stewards” of antibiotic prescribing, this problem would be significantly minimized.
The development of new drugs and diagnostic tests, core action four, is vitally important. Bacteria will always evolve and become resistant, so it is important to continually develop new antibiotics and diagnostic tests to track antibiotic resistance. The more an antibiotic is used, the faster it develops bacterial resistance. Antibiotics are a limited resource and must be treated carefully.4
The comprehensive CDC report lists each of the bacterial threats in the urgent, serious, and concerning categories, and provides a fact sheet for each. The fact sheet contains a brief description of the bacteria, resistance of concern, level of public health threat, statistics, the actions the CDC has taken, and what the public and health care workers can do to address these threats. Online resources are also available. The CDC report includes a technical appendix for each bacterial threat, including further statistics, research findings, and bibliographic references.4 All health care workers should be familiar with this document.
WORLD HEALTH ORGANIZATION DOCUMENT
The WHO has also developed a guidance document to aid in combating AMR titled “WHO Global Strategy for Containment of Antimicrobial Resistance.”3 The report states that “a global problem requires a global response.” The response was the development of a framework to raise awareness, promote information sharing, provide guidance on interventions to address AMR, assist various nations in their efforts, and stimulate research related to AMR. The report contains three sections: background information about AMR; interventions for patients/community, prescribers, hospitals, food chain, government health systems, drug development, pharmaceutical promotion, and international perspectives; and implementation strategies to fight AMR. The report encourages collaboration among governments, professional associations, and international agencies to recognize the significance of AMR and implement strategies to contain it. The report also encourages establishment of programs for epidemiological surveillance and an international database of research funding and programs targeted at AMR.3
WHAT HEALTH CARE PROFESSIONALS CAN DO
The CDC guidance for antibiotic stewardship in hospitals includes several elements that personnel should follow. These include: a single physician should supervise each patient’s care for accountability purposes; drug expertise and medication management should occur through a single pharmacy contact; interventions, such as periodic breaks from antibiotics, should be implemented to improve prescribing practices; tracking and reporting of prescribing and resistance patterns need to happen; and clinicians should seek out continuing education on AMR.6 Health care leaders need to support the CDC’s recommendations and work to ensure they are properly implemented.
Similar recommendations from the WHO report include educating patients about the appropriate use of antibiotics, hand washing and other infection prevention measures, and discussion on alternatives to antibiotics for relief of symptoms. The WHO also recommends targeting education programs about AMR to undergraduate students in various health professions, as well as continuing education courses designed for practicing clinicians.3
WHY DENTAL PROFESSIONALS SHOULD BE CONCERNED
Dental professionals play a vital role in combating antibiotic resistance. Educating the dental community and dental patients about the problem of antibiotic resistance is an important step in becoming good stewards. Posting signage about hand washing and other routine infection prevention methods and discussing appropriate antibiotic use are some strategies oral health professionals can employ. The dental community must be diligent in educating patients and prescribing antibiotics only when it is truly necessary. Following the core actions in the CDC report and implementing the WHO document strategies will help bolster the fight against antibiotic resistance.
REFERENCES
- Centers for Disease Control and Prevention. Get Smart Fact Sheet: Know When Antibiotics Work. Available at: cdc.gov/getsmart/antibiotic-use/antibiotic-resistance-faqs.html. Accessed March 21, 2014.
- Lewis R. The rise of antibiotic-resistant infections. FDA Consum. 1995;29:11–15.
- World Health Organization. WHO Global Strategy for Containment of Antimicrobial Resistance. Available at: who.int/drugresistance/ WHO_Global_Strategy.htm/en. Accessed March 21, 2014.
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013 Report 2013. Available at: cdc.gov/ drugresistance/threat-report-2013. Accessed March 21, 2014.
- Centers for Disease Control and Prevention. About CDC: Mission Role and Pledge. Available at: cdc.gov/about/organization/mission.htm. Accessed March 21, 2014.
- Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63:194–200.
- Hughes JM. Preserving the lifesaving power of antimicrobial agents. JAMA. 2011;305:1027–1028.
- Vernet G, Mary C, Altmann DM, et al. Surveillance for antimicrobial drug resistance in under-resourced countries. Emerg Infect Dis. 2014;20:434–441.
- Hecker MT, Aron DC, Patel NP, Lehmann MK, Donskey CJ. Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med. 2003;163:972–978.
- Macfarlane J, Holmes W, Macfarlane R, Britten N. Influence of patients’ expectations on antibiotic management of acute lower respiratory tract illness in general practice: questionnaire study. BMJ. 1997;315:1211–1214.
- Wendt JM, Cohen JA, Mu Y, et al. Clostridium difficile infection among children across diverse US geographic locations. Pediatrics. 2014;133:1–8.
- Bonomo RA. Multiple antibiotic resistant bacteria in long-term-care facilities: an emerging problem in the practice of infectious disease. Clin Infect Dis. 2000;31:1414–1422.
- Stone PW, Pogorzelska-Maziarz M, Herzig CT, et al. State of infection prevention in US hospitals enrolled in the National Health and Safety Network. Am J Infect Control. 2014;42:94–99.
From Dimensions of Dental Hygiene. April 2014;12(4):48–52.



