
Enamel Revisited
How enamel creates a beautiful smile and has the possibility of regeneration.
in both personal and professional lives. In fact, the reflection of our smile from a mirror often creates a sense of well-being and supports our sense of internal value. But our smile is so much more. Our smile can reflect intent, appreciation, and, most important, smiling reveals one of the most beautiful tissues found in the human body—the magnificent enamel covering the surfaces of our teeth.
Amelogenesis is the process by which ectodermally-derived ameloblast cells synthesize and secrete enamel matrix proteins which, in turn, seed or nucleate and regulate the formation of calcium hydroxyapatite crystals. The final product is enamel—the hardest tissue in the vertebrate body. Giving the gift of a smile is one of the most rewarding parts of the oral health professions.
THE WONDERS OF ENAMEL
The enamel tissue forms in juxtaposition to dentin (without an intervening tissue or boundary). Enamel initially consists of an organic extracellular matrix composed of a number of different types of proteins. Within the protein environment, calcium hydroxyapatite crystals grow along their individual c-axis. These crystals eventually assemble into a remarkable bioceramic that becomes the hardest tissue in the body.
Following enamel formation and biomineralization, the formative amelobast cells are reduced and eventually form a pellicle on the surfaces of erupting teeth. After tooth eruption, there are no more cells available for the replacement or regeneration of enamel. The extraordinary assembly of enamel crystals and calcium hydroxyapatite within the enamel tissue that covers the teeth provides the unique substrate for light, contributing to the dazzling splendor of a full smile.
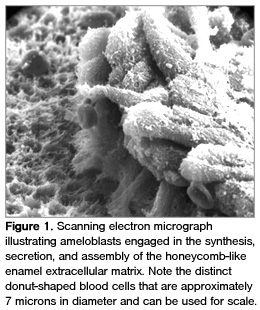
 Amelogenesis describes the entire biological process of producing enamel. In human development, it begins during the seventh month of pregnancy or the beginning of the third trimester on the deciduous mandibular incisors and is completed by 6 months postnatally. The process entails the building of an extracellular matrix composed of various proteins, ie, amelogenins, ameloblastins, metalloproteinases, and other enzymes; glycosaminoglycans; minerals; and water (Figure 1). The major protein is amelogenin and the human gene is located on both the X and Y chromosomes.1,2
Amelogenesis describes the entire biological process of producing enamel. In human development, it begins during the seventh month of pregnancy or the beginning of the third trimester on the deciduous mandibular incisors and is completed by 6 months postnatally. The process entails the building of an extracellular matrix composed of various proteins, ie, amelogenins, ameloblastins, metalloproteinases, and other enzymes; glycosaminoglycans; minerals; and water (Figure 1). The major protein is amelogenin and the human gene is located on both the X and Y chromosomes.1,2
The logic of the enamel-forming microenvironment is to seed or nucleate mineral deposition at a specific location and create a nanoenvironment that enables crystal formation and crystal growth essentially in the c-axis (long axis) in order to produce the longest and largest crystals in all of biology—the enamel bioceramic, which grows along the dentin-enamel junction. Amelogenins form nanospheres, and within these environs, crystal growth is initiated.3-6
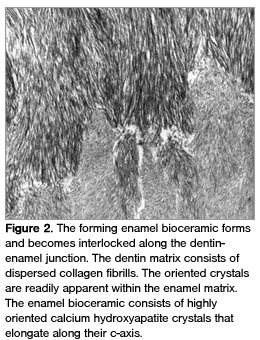
While the multiple protein-protein interactions mediate crystal growth, eg, charge distribution on the amino acids that constitute the protein, pH, etc, proteins are degraded by enzymes (metalloproteinases) to result in the fully mineralized enamel ceramic (Figure 2).
In tandem, dentinogenesis is a complementary process that builds an extracellular matrix, primarily using type I and III collagens along with sialoglycoproteins and phosphoproteins. The dentin proteins are not degraded, and the small crystals that are formed within dentin create yet another type of bioceramic termed dentin. Dentin forms in advance of enamel and then enamel forms along the dentin surface, which is termed the dentin-enamel junction. Importantly, during the earliest stages of both the ectodermally-derived amelogenesis and the mesenchymal-derived dentinogenesis, active cell-cell communications between disparate tissue types is regulated by a number of intriguing molecules termed growth factors.7
Curiously, as the crown of the tooth is being completed, Hertwig’s Epithelial Root Sheath (HERS) forms and advances apically to draft the outline for root formation while differentiating into various cell types associated with the formation of acellular cementum. Many years ago, it was postulated that cementum contained enamel-like proteins, which had antigenic determinants that induce antibodies directed against amelogenin.8 Thereafter, a number of other scientists learned that HERS contain messenger ribonucleic acids (RNAs) that are related to amelogenins and that cementum contains types 1 and III collagens and trace amounts of enamel-related proteins.7,9,10-13
 Further analyses also suggest that various growth factors are found within extracellular matrices, which are associated with biomineralization of enamel, dentin, and cementum as well as bone and cartilage.7,14,15
Further analyses also suggest that various growth factors are found within extracellular matrices, which are associated with biomineralization of enamel, dentin, and cementum as well as bone and cartilage.7,14,15
ENAMEL AS A THERAPEUTIC AGENT?
Based on the intriguing developmental biology of tooth development, especially root formations, several scientists postulated that the enamel extracellular organic matrix contained informative molecules that possibly could induce new periodontal tissue formation following periodontal destruction. Perhaps amelogenin or other enamel-related proteins (even possible growth factors) might induce new tissues. The challenge was to create periodontal tissue lesions in animal models and to test the hypothesis that enamel-related proteins induce new tissues and potentially regenerate the periodontium.
10,11,16 The commercial product changed the spelling to Emdogain®*. It is made from lyophilized bovine enamel extracellular matrix proteins. The claim is that the organic enamel matrix can function as an inducer of periodontal tissue regeneration, ie, cementum, Sharpey’s fibers, and alveolar bone.7,16
ENAMEL REGENERATION
From another perspective, numerous scientific groups have postulated that enamel can be repaired or regenerated using recombinant DNA technology. Enamel protein genes have been identified, cloned, and sequenced.1,4 A number of these genes are involved with enamel biomineralization. Techniques are available to place human genes into bioreactors that can yield kilograms of highly specific proteins. These enamel recombinant proteins (amelogenins, ameloblastins, etc) can be used to form an enamel paste that can be applied to enamel defects.17
SUMMARY
Scientific discovery produces the fuel that powers the engine of technology and applications to clinical dentistry.18 The completion of the human genome in October 2004 led to our understanding of biological mechanisms required for morphogenesis (how we form tissues, organs, and systems of the body); the RNAs that form the human transcriptomes; the proteins that comprise the human proteome; and the various life-supporting processes that form the human metabolome (the complete complement of all small molecule chemicals found in or produced by an organism). Our future in clinical dentistry is enormously promising and bright.
REFERENCES
- Lau EC, Mohandas TK, Shapiro LJ, Slavkin HC, Snead ML. Human and mouse amelogenin gene loci are on the sex chromosomes. Genomics. 1989;4:162-168.
- Fincham AG, Hu Y, Lau EC, Slavkin HC, Snead ML. Amelogenin post-secretory processing during biomineralization in the postnatal mouse molar tooth. Arch Oral Biol. 1991;36:305-317.
- Fincham AG, Moradian-Oldak J, Simmer JP, et al. Self-assembly of a recombinant amelogenin protein generates supramolecular structures. J Struct Biol. 1994;112:103-109.
- Fincham AG, Moradian-Oldak J, Simmer JP. The structural biology of the developing dental enamel matrix. J Struct Biol. 1999;126:270-299.
- Moradian-Oldak J, Tan J, Fincham AG. Interaction of amelogenin with hydroxyapatite crystals: an adherence effect through amelogenin molecular self-association. Biopolymers. 1998;46:225-238.
- Moradian-Oldak J, Simmer JP, Lau EC, Sarte PE, Slavkin HC, Fincham AG. Detection of monodisperse aggregates of a recombinant amelogenin by dynamic light scattering. Biopolymers. 1994;34:1339-1347.
- Zeichner-David M. Regeneration of periodontal tissues: cementogenesis revisited. Periodontol 2000. 2006;41:196-217.
- Slavkin HC. Towards a cellular and molecular understanding of periodontics. Cementogenesis revisited. J Perio. 1976;47:249-255.
- Slavkin HC, Bessem C, Fincham AG, et al. Human and mouse cementum proteins immunologically related to enamel proteins. Biochimica Et Biophysica Acta. 1989;991:12-18.
- Hammarstrom L. Enamel matrix, cementum development and regeneration. J Clin Perio.1997;24:658-668.
- Hammarstrom L. The role of enamel matrix proteins in the development of cementum and periodontal tissues. Ciba Found Symp. 1997;205:246-255;discussion 255-260.
- Lindskog S, Hammarstrom L. Formation of intermediate cementum. III: 3H-tryptophan and 3H-proline uptake into the epithelial root sheath of Hertwig in vitro. J Craniofac Genet Dev Biol. 1982;2:171-177.
- Lindskog S, Blomlof L, Hammarstrom L. Evidence for a role of odontogenic epithelium in maintaining the periodontal space. J Clin Perio. 1988;15:371-373.
- Diekwisch T, David S, Bringas P, Jr., Santos V, Slavkin HC. Antisense inhibition of AMEL translation demonstrates supramolecular controls for enamel HAP crystal growth during embryonic mouse molar development. Development. 1993;117:471-482.
- Alvarez-Perez MA, Narayanan S, Zeichner-David M, Rodriguez Carmona B, Arzate H. Molecular cloning, expression and immunolocalization of a novel human cementum-derived protein (CP-23). Bone. 2006;38:409-419.
- He J, Jiang J, Safavi KE, Spangberg LS, Zhu Q. Emdogain promotes osteoblast proliferation and differentiation and stimulates osteoprotegerin expression. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:239-245.
- Yamagishi K, Onuma K, Suzuki T, Okada F, Tagami J, Otsuki M, Senawangse P. Materials chemistry: a synthetic enamel for rapid tooth repair. Nature. 2005;433:819.
- Moradian-Oldak J, Wen HB, Schneider GB, Stanford CM. Tissue engineering strategies for the future generation of dental implants. Periodontol 2000. 2006;41:157-176.
From Dimensions of Dental Hygiene. June 2007;5(6): 16-18.

