
Contaminated Dental Aerosols
The risks and implications for dental hygienists.
Dental hygienists practice in a highly contaminated environment—the human mouth. The mouth contains bacteria and viruses from many sources. The saliva of a healthy patient contains large numbers of streptococci, staphylococci, and gram negative bacteria. While many of these organisms are relatively innocuous for a healthy person, they carry the possibility of infection for the increasing number of people with compromised immune systems.
Of more concern to hygienists are the pathogenic bacteria and viruses originating from nonsalivary and nontooth origins. These include organisms from the nasopharynx, sputum originating in the lungs, and blood routinely encountered during root planing. These areas are the sources of many pathogenic organisms such as tuberculosis (TB), hepatitis, human immunodeficiency virus (HIV), and severe acute respiratory syndrome (SARS).
The Nature of Dental Aerosols
Most procedures performed by dental hygienists have the potential for creating contaminated aerosols and splatter. The use of hand scalers, prophy angles, and air-water syringes produce some splatter in the form of relatively large droplets. While large droplets contain potentially pathogenic organisms, they are usually controlled by standard barrier techniques such as gloves, masks, and eye protection. However, the ultrasonic scaler and the air polisher are the greatest producers of small particle aerosol contamination in dentistry.1-6 Several studies1-6 show that the ultrasonic scaler produces more airborne contamination than any other instrument in dentistry. The air polisher creates an almost equal amount of airborne contamination. The use of these instruments places hygienists at the forefront of risk for the airborne transmission of infections.
Many hygienists assume that the visible aerosols produced by an ultrasonic scaler or an air polisher are the only aerosols produced by these instruments. These highly visible aerosols are made up of coolant water and, in the case of an air polisher, some form of abrasive. If the instrument and the dental unit water lines are maintained as recommended by the American Dental Association (ADA),7 the coolant water aerosol represents little danger to the operator. The risk of infection occurs when these instruments are used on a patient and the visible aerosol mixes with the invisible microorganisms that arise from the patient.
.jpg) |
Figure 1. Visible coolant water aerosol created by a straight style ultrasonic insert using the standard 17ml/min of coolant water. The actual contaminated aerosol produced during clinical use is much more extensive than the visible aerosol pictured here. |
.jpg) |
Figure 2. Visible aerosol produced by a straight “slim” style insert using a standard 17ml/min volume of coolant water. The use of a saliva ejector has almost no effect on the aerosol produced even when placed at a much more favorable position (approximately 1 cm from the ultrasonic tip) than is the case clinically. A saliva ejector is ineffective in reducing aerosols. |
.jpg) |
Figure 3. The visible aerosol produced by the same tip and coolant water volume pictured in Figure 1 with a high volume evacuator attached to the handle of the ultrasonic scaler. This high volume evacuation device reduces both coolant water aerosol and bacterial contamination by 95%. |
An in vitro study evaluated this risk potential by measuring the aerosol that came from the operating site.8 In this study, an ultrasonic scaler was used for 3 seconds without coolant water so there was no coolant water aerosol. The tip of the ultrasonic scaler was placed in a small amount of fluorescent fluid equivalent to one drop of saliva or blood. The aerosol produced was evaluated by analyzing the amount of fluorescent contamination present after 3 seconds of use. Hundreds of fluorescent droplets spread over greater than 18 inches were detected. The aerosol produced in this study was virtually invisible to the naked eye. However, this invisible aerosol is the source of infection risk for dental hygienists, not the highly visible aerosols from coolant water.
While studies have evaluated the number of organisms propelled into the air by various dental instruments, most of these studies counted organisms that grew on a petri dish exposed to the air of the operatory. Unfortunately, due to the nature of the culture medium used, these studies only counted the relatively innocuous aerobic bacteria in the aerosol. They did not count the number of anaerobic bacteria, such as those found in periodontal pockets; the number of difficult to culture bacteria, such as TB; or the number of viruses. These organisms are much more likely to be pathogenic and represent the real danger.
Potential Danger
Scientific literature detailing the spread of infection by an aerosol route is available. Recent examples include the spread of TB on an airplane and the spread of measles through the ventilation system of a pediatric office.9-10 Traditionally, the greatest danger inherent in dental aerosols was TB. In the past, TB was considered an occupational hazard for dentistry. Currently in the United States, TB is rare. However, in certain populations such as recent immigrants, prisons, and the homeless there is a much higher prevalence of TB. Hygienists working with patients from these groups need to approach treatment with great care due to the potential for undiagnosed TB. In private clinical practice, the greatest danger comes from viruses harbored in the nasopharynx and blood particles arising from the operative site.
Viruses
Many viruses are present in the mouth originating from the saliva, gingival tissues, and from the nose, throat, and lungs. These include the common cold, influenza, and herpetic viruses. The greatest current concern is the SARS virus. The only report of a dental professional who contracted SARS was an Associated Press report of a Taiwanese dentist who died from the disease.11 SARS is apparently caused by a coronavirus similar to the viruses that cause the common cold. Hygienists have a 60% higher incidence of cold symptoms than a similar professional group without dental patient contact.12 It appears that a risk of coronavirus transmission between patient and practitioner may exist. The Centers for Disease Control and Prevention (CDC) and the ADA have recommended that all procedures generating aerosols be avoided in the presence of SARS because it can be spread by contaminated aerosols.13-14 Because a patient with active SARS is extremely ill, it is unlikely that any dental procedures would be performed. However, the incubation time for SARS is 2 to 10 days and possibly longer. There is a potential for SARS to be transmitted during this incubation stage or possibly during a carrier state that may exist for an extended period of time after recovery. Due to this potential risk, as with HIV, hygienists should follow universal precautions for aerosols on the assumption that all patients carry an infectious disease that can be spread by an aerosol route.
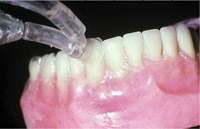 |
Figure 4. An aerosol reduction device (JetShield™ Cavitron®) being used during air polishing. This device will reduce aerosol and bacterial contamination by 97%. A high volume evacuator is ineffective for controlling the aerosol from an air polisher due to the ricochet phenomena. |
 |
Figure 5. The visible aerosol produced by an air polisher (Prophy-Jet® Cavitron®). Both abrasive particles and water droplets are propelled forcefully against the tooth by compressed air. This force will cause the water and abrasive to ricochet off of the tooth surface and into the air. |
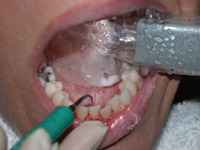 |
Figure 6. A “dry field” device (Isolite™ Isolite Systems) used during scaling with an ultrasonic scaler. These devices place an HVE in the operative field. Research is needed to determine to what extent these devices will reduce aerosols. |
Blood
Several studies have shown that blood is universally found in the aerosols produced by an ultrasonic scaler during root planing.15,16 These studies further demonstrate that even when the operator cannot see blood contamination there is an adequate amount of blood in the aerosols from root planing to be detected by an occult blood test. This is true no matter what type of ultrasonic scaler or scaler tip is used. The use of ultrasonic and sonic vibration in the presence of a liquid, ie, blood, aerosolizes the liquid. Whatever bloodborne infection the patient may carry is probably present in the dental aerosols from root planing with an ultrasonic scaler.
Preventative Measures
Gloves, well-fitting masks, and eye protection should always be used and greatly lowers the risk of infection. However, a true aerosol stays airborne for up to 30 minutes and larger droplets evaporate and the contamination they carry can become re-airborne as a dust particle, ie, a droplet nuclei. Therefore, the risk of contamination continues long after the procedure is over. Because of this extended risk time as well as the often poor fit of masks and eye protection, other precautions should be followed. Using a preprocedural rinse such as chlorhexidine lowers the number of aerobic bacteria in the air. However, it will not affect blood coming from the operative site or viruses, such as SARS, coming from the respiratory tract. Using a preprocedural rinse should not be relied on to prevent airborne contamination.
The use of a high volume evacuator (HVE) has been shown to universally reduce airborne contamination, no matter what the dental source by 90% to 98%.17-19 However, a saliva ejector does not qualify as a high volume evacuator. The small diameter of a saliva ejector keeps it from removing enough air to be effective in reducing aerosols. A large diameter HVE may be held separately by an assistant or the hygienist, attached to the ultrasonic scaler, or possibly attached to one of the “dry field” devices. Using an HVE is a mandatory infection control precaution during the use of an ultrasonic scaler.
The aerosols from air polishing are more difficult to control than those from an ultrasonic scaler due to the compressed air used during air polishing. The only method proven to control the aerosols from air polishing combines a barrier placed around the air polishing tip combined with a vacuum.20 The barrier blocks the high speed particles from ricocheting off the tooth into the air and the vacuum removes the water and abrasive particles from the operating site within the barrier. This method has been shown to reduce the airborne contamination from air polishing by 97%.6,20
Summary
Dental aerosols represent an infection hazard for dental hygienists due to their gross contamination with microorganisms and blood. The advent of SARS and its predicted reemergence during the upcoming flu season have brought the dangers of dental aerosols to a higher level. Aerosols are easily controlled with the appropriate precautions. Whenever an ultrasonic scaler or air polisher is used the following steps should be followed: (1) barrier protection (2) high volume evacuation, and (3) preprocedural rinsing. Each of these adds a layer of protection for the operator and others in the dental office. All three steps must be followed for adequate protection. The use of only one or two of the steps will not yield the necessary level of protection adequate for safety.
References
- King TB, Muzzin KB, Berry CW, Anders LM. The effectiveness of an aerosol reduction device for ultrasonic scalers. J Periodontol. 1997; 68:45-49.
- Logothetis DD, Gross KB, Eberhart A, Drisko C. Bacterial airborne contamination with an air-polishing device. Gen Dent. 1988;36(6):496-499.
- Bentley CD, Burkhart NW, Crawford JJ. Evaluating spatter and aerosol contamination during dental procedures. J Am Dent Assoc. 1994;125:579-584.
- Legnani P, Checchi L, Pelliccioni GA, D’Achille C. Atmospheric contamination during dental procedures. Quintessence Int. 1994;25:435-439.
- Gross KB, Overman PR, Cobb C, Brockman S. Aerosol generation by two ultrasonic scalers and one sonic scaler. A comparative study. J Dent Hyg. 1992;66:314-318.
- Muzzin KB, King TB, Berry CW. Assessing the clinical effectiveness of an aerosol reduction device for the air polisher. J Am Dent Assoc. 1999;130:1354-1359.
- Murdoch-Kinch CA, Andrews NL, Atwan S, Jude R, Gleason MJ, Molinari JA. Comparison of dental water quality management procedures. J Am Dent Assoc. 1997;128:1235-1243.
- Harrel SK, Barnes JB, Rivera-Hidalgo F. Aerosol and splatter contamination from the operative site during ultrasonic scaling. J Am Dent Assoc. 1998;129:1241-1249.
- Kenyon TA, Valway SE, Ihle WW, Onorato IM, Castro KG. Transmission of multidrug-resistant Mycobacterium tuberculosis during a long airplane flight. N Engl J Med. 1996;334(15):933-938.
- Bloch AB, Orenstein WA, Ewing WM, et al. Measles outbreak in a pediatric practice: airborne transmission in an office setting. Pediatrics. 1985;75:676-683.
- Associated Press News Service. SARS Surges in Taiwan. Available at: www.foxnews.com/printer_friendly_story/0,3566,86599,00.html. Accessed August 6, 2003.
- Rosen S, Schmakel D, Schoener M. Incidence of respiratory disease in dental hygienists and dietitians. Clin Prevent Dent. 1985; 7:24-25.
- CDC. Interim Domestic Infection Control Precautions for Aerosol-Generating Procedures on Patients with Severe Acute Respiratory Syndrome. Available at: www.cdc.gov/ncidod/sars /aerosolinfectioncontrol.htm. Accessed June 14, 2003.
- American Dental Association. Severe Acute Respiratory Syndrome. Available at: www.ada.org/prof/prac/issues/topics/sars.html. Accessed June 14, 2003.
- Harrel SK. Clinical use of an aerosol-reduction device with an ultrasonic scaler. Compend Contin Educ Dent. 1996;17:1185-1193.
- Barnes JB, Harrel SK, Rivera-Hidalgo F. Blood contamination of the aerosols produced by the in vivo use of ultrasonic scalers. J Periodontol. 1998;69:434-438.
- Harrel SK, Barnes JB, Rivera-Hidalgo F. Reduction of aerosols produced by ultrasonic scalers. J Periodontol. 1996;67:28-32.
- Jacks ME. A laboratory comparison of evacuation devices on aerosol reduction. J Dent Hyg. 2002;76:202-206.
- Klyn SL, Cummings DE, Richardson BW, Davis RD. Reduction of bacteria-containing spray produced during ultrasonic scaling. Gen Dent. 2001;49(6):648-652.
- Harrel SK, Barnes JB, Rivera-Hidalgo F. Aerosol reduction during air polishing. Quintessence Int. 1999;30:623-628.
From Dimensions of Dental Hygiene. October 2003;1(6):16, 18, 20.

